Mapping the intermedilysin-human CD59 receptor interface reveals a deep correspondence with the binding site on CD59 for complement binding proteins C8alpha and C9
- PMID: 21507937
- PMCID: PMC3121471
- DOI: 10.1074/jbc.M111.237446
Mapping the intermedilysin-human CD59 receptor interface reveals a deep correspondence with the binding site on CD59 for complement binding proteins C8alpha and C9
Abstract
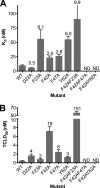
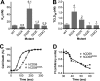
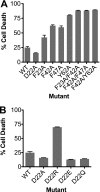
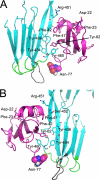
CD59 is a glycosylphosphatidylinositol-anchored protein that inhibits the assembly of the terminal complement membrane attack complex (MAC) pore, whereas Streptococcus intermedius intermedilysin (ILY), a pore forming cholesterol-dependent cytolysin (CDC), specifically binds to human CD59 (hCD59) to initiate the formation of its pore. The identification of the residues of ILY and hCD59 that form their binding interface revealed a remarkably deep correspondence between the hCD59 binding site for ILY and that for the MAC proteins C8α and C9. ILY disengages from hCD59 during the prepore to pore transition, suggesting that loss of this interaction is necessary to accommodate specific structural changes associated with this transition. Consistent with this scenario, mutants of hCD59 or ILY that increased the affinity of this interaction decreased the cytolytic activity by slowing the transition of the prepore to pore but not the assembly of the prepore oligomer. A signature motif was also identified in the hCD59 binding CDCs that revealed a new hCD59-binding member of the CDC family. Although the binding site on hCD59 for ILY, C8α, and C9 exhibits significant homology, no similarity exists in their binding sites for hCD59. Hence, ILY and the MAC proteins interact with common amino acids of hCD59 but lack detectable conservation in their binding sites for hCD59.
Figures



References
-
- Law R. H., Lukoyanova N., Voskoboinik I., Caradoc-Davies T. T., Baran K., Dunstone M. A., D'Angelo M. E., Orlova E. V., Coulibaly F., Verschoor S., Browne K. A., Ciccone A., Kuiper M. J., Bird P. I., Trapani J. A., Saibil H. R., Whisstock J. C. (2010) Nature 468, 447–451 - PubMed
-
- Rosado C. J., Buckle A. M., Law R. H., Butcher R. E., Kan W. T., Bird C. H., Ung K., Browne K. A., Baran K., Bashtannyk-Puhalovich T. A., Faux N. G., Wong W., Porter C. J., Pike R. N., Ellisdon A. M., Pearce M. C., Bottomley S. P., Emsley J., Smith A. I., Rossjohn J., Hartland E. L., Voskoboinik I., Trapani J. A., Bird P. I., Dunstone M. A., Whisstock J. C. (2007) Science 317, 1548–1551 - PubMed
-
- Hadders M. A., Beringer D. X., Gros P. (2007) Science 317, 1552–1554 - PubMed
-
- Shatursky O., Heuck A. P., Shepard L. A., Rossjohn J., Parker M. W., Johnson A. E., Tweten R. K. (1999) Cell 99, 293–299 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

