Platelet glycoprotein Ib beta/IX mediates glycoprotein Ib alpha localization to membrane lipid domain critical for von Willebrand factor interaction at high shear
- PMID: 21507943
- PMCID: PMC3122191
- DOI: 10.1074/jbc.M110.202549
Platelet glycoprotein Ib beta/IX mediates glycoprotein Ib alpha localization to membrane lipid domain critical for von Willebrand factor interaction at high shear
Abstract
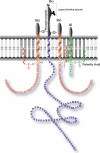
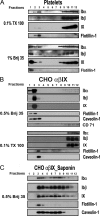
The localization of the platelet glycoprotein GP Ib-IX complex (GP Ibα, GP Ibβ, and GP IX) to membrane lipid domain, also known as glycosphingolipid-enriched membranes (GEMs or raft) lipid domain, is essential for the GP Ib-IX complex mediated platelet adhesion to von Willebrand factor (vWf) and subsequent platelet activation. To date, the mechanism for the complex association with the GEMs remains unclear. Although the palmitate modifications of GP Ibβ and GP IX were thought to be critical for the complex presence in the GEMs, we found that the removal of the putative palmitoylation sites of GP Ibβ and GP IX had no effects on the localization of the GP Ib-IX complex to the GEMs. Instead, the disruption of GP Ibα disulfide linkage with GP Ibβ markedly decreased the amount of the GEM-associated GP Ibα without altering the GEM association of GP Ibβ and GP IX. Furthermore, partial dissociation with the GEMs greatly inhibited GP Ibα interaction with vWf at high shear instead of in static condition or under low shear stress. Thus, for the first time, we demonstrated that GP Ibβ/GP IX mediates the disulfide-linked GP Ibα localization to the GEMs, which is critical for vWf interaction at high shear.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

