Charge requirements for proton gradient-driven translocation of anthrax toxin
- PMID: 21507946
- PMCID: PMC3123086
- DOI: 10.1074/jbc.M111.231167
Charge requirements for proton gradient-driven translocation of anthrax toxin
Abstract
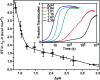
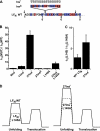
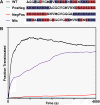
Anthrax lethal toxin is used as a model system to study protein translocation. The toxin is composed of a translocase channel, called protective antigen (PA), and an enzyme, called lethal factor (LF). A proton gradient (ΔpH) can drive LF unfolding and translocation through PA channels; however, the mechanism of ΔpH-mediated force generation, substrate unfolding, and establishment of directionality are poorly understood. One recent hypothesis suggests that the ΔpH may act through changes in the protonation state of residues in the substrate. Here we report the charge requirements of LF's amino-terminal binding domain (LF(N)) using planar lipid bilayer electrophysiology. We found that acidic residues are required in LF(N) to utilize a proton gradient for translocation. Constructs lacking negative charges in the unstructured presequence of LF(N) translocate independently of the ΔpH driving force. Acidic residues markedly increase the rate of ΔpH-driven translocation, and the presequence is optimized in its natural acidic residue content for efficient ΔpH-driven unfolding and translocation. We discuss a ΔpH-driven charge state Brownian ratchet mechanism for translocation, where glutamic and aspartic acid residues in the substrate are the "molecular teeth" of the ratchet. Our Brownian ratchet model includes a mechanism for unfolding and a novel role for positive charges, which we propose chaperone negative charges through the PA channel during ΔpH translocation.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

