The single RBP-Jkappa site within the LANA promoter is crucial for establishing Kaposi's sarcoma-associated herpesvirus latency during primary infection
- PMID: 21507979
- PMCID: PMC3126528
- DOI: 10.1128/JVI.02608-10
The single RBP-Jkappa site within the LANA promoter is crucial for establishing Kaposi's sarcoma-associated herpesvirus latency during primary infection
Abstract
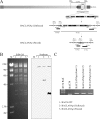
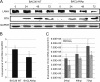
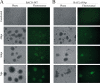
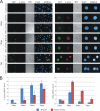
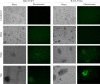
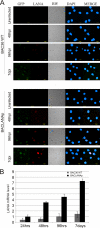
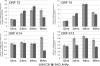
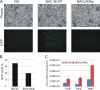
Kaposi's sarcoma-associated herpesvirus (KSHV; or human herpesvirus 8 [HHV8]) is implicated in the pathogenesis of many human malignancies including Kaposi's sarcoma (KS), multicentric Castleman's disease (MCD), and primary effusion lymphoma (PEL). KSHV infection displays two alternative life cycles, referred to as the latent and lytic or productive cycle. Previously, we have reported that the replication and transcription activator (RTA), a major lytic cycle transactivator, contributes to the development of KSHV latency by inducing latency-associated nuclear antigen (LANA) expression during early stages of infection by targeting RBP-Jκ, the master regulator of the Notch signaling pathway. Here, we generated a bacterial artificial chromosome (BAC) KSHV recombinant virus with a deletion of the RBP-Jκ site within the LANA promoter to evaluate the function of the RBP-Jκ cognate site in establishing primary latent infection. The results showed that genetic disruption of the RBP-Jκ binding site within the KSHV LANA promoter led to enhanced expression of the KSHV-encoded immediate early RTA, resulting in an increase in lytic replication during primary infection of human peripheral blood mononuclear cells (PBMCs). This system provides a powerful tool for use in indentifying additional cellular and viral molecules involved in LANA-mediated latency maintenance during the early stages of KSHV infection.
Figures

References
-
- An F. Q., et al. 2005. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus modulates cellular gene expression and protects lymphoid cells from p16 INK4A-induced cell cycle arrest. J. Biol. Chem. 280:3862–3874 - PubMed
-
- Barcy S., et al. 2008. Gamma delta+ T cells involvement in viral immune control of chronic human herpesvirus 8 infection. J. Immunol. 180:3417–3425 - PubMed
-
- Boivin G., Gaudreau A., Routy J. P. 2000. Evaluation of the human herpesvirus 8 DNA load in blood and Kaposi's sarcoma skin lesions from AIDS patients on highly active antiretroviral therapy. AIDS 14:1907–1910 - PubMed
-
- Brown E. E., et al. 2006. Virologic, hematologic, and immunologic risk factors for classic Kaposi sarcoma. Cancer 107:2282–2290 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

