Different pain, different brain: thalamic anatomy in neuropathic and non-neuropathic chronic pain syndromes
- PMID: 21508220
- PMCID: PMC6632967
- DOI: 10.1523/JNEUROSCI.5980-10.2011
Different pain, different brain: thalamic anatomy in neuropathic and non-neuropathic chronic pain syndromes
Abstract
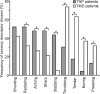
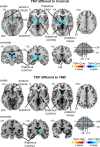
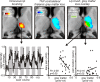
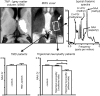
Trigeminal neuropathic pain (TNP) and temporomandibular disorders (TMD) are thought to have fundamentally different etiologies. It has been proposed that TNP arises through damage to, or pressure on, somatosensory afferents in the trigeminal nerve, whereas TMD results primarily from peripheral nociceptor activation. Because some reports suggest that neuropathic pain is associated with changes in brain anatomy, it is possible that TNP is maintained by changes in higher brain structures, whereas TMD is not. The aim of this investigation is to determine whether changes in regional brain anatomy and biochemistry occur in both conditions. Twenty-one TNP subjects, 20 TMD subjects, and 36 healthy controls were recruited. Voxel-based morphometry of T1-weighted anatomical images revealed no significant regional gray matter volume change in TMD patients. In contrast, gray matter volume of TNP patients was reduced in the primary somatosensory cortex, anterior insula, putamen, nucleus accumbens, and the thalamus, whereas gray matter volume was increased in the posterior insula. The thalamic volume decrease was only seen in the TNP patients classified as having trigeminal neuropathy but not those with trigeminal neuralgia. Furthermore, in trigeminal neuropathy patients, magnetic resonance spectroscopy revealed a significant reduction in the N-acetylaspartate/creatine ratio, a biochemical marker of neural viability, in the region of thalamic volume loss. The data suggest that the pathogenesis underlying neuropathic and non-neuropathic pain conditions are fundamentally different and that neuropathic pain conditions that result from peripheral injuries may be generated and/or maintained by structural changes in regions such as the thalamus.
Figures


Comment in
-
Making sense of gray matter abnormalities in chronic orofacial pain--synthesizing divergent findings.J Neurosci. 2011 Aug 31;31(35):12396-7. doi: 10.1523/JNEUROSCI.3103-11.2011. J Neurosci. 2011. PMID: 21880900 Free PMC article. No abstract available.
References
-
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. - PubMed
-
- Banati RB. Brain plasticity and microglia: is transsynaptic glial activation in the thalamus after limb denervation linked to cortical plasticity and central sensitisation? J Physiol Paris. 2002;96:289–299. - PubMed
-
- Banati RB, Cagnin A, Brooks DJ, Gunn RN, Myers R, Jones T, Birch R, Anand P. Long-term trans-synaptic glial responses in the human thalamus after peripheral nerve injury. Neuroreport. 2001;12:3439–3442. - PubMed
-
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
