Nutrient-sensing hypothalamic TXNIP links nutrient excess to energy imbalance in mice
- PMID: 21508227
- PMCID: PMC3100164
- DOI: 10.1523/JNEUROSCI.6498-10.2011
Nutrient-sensing hypothalamic TXNIP links nutrient excess to energy imbalance in mice
Abstract
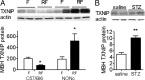
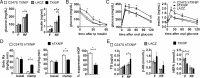
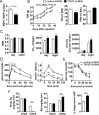
Nutrient excess in obesity and diabetes is emerging as a common putative cause for multiple deleterious effects across diverse cell types, responsible for a variety of metabolic dysfunctions. The hypothalamus is acknowledged as an important regulator of whole-body energy homeostasis, through both detection of nutrient availability and coordination of effectors that determine nutrient intake and utilization, thus preventing cellular and whole-body nutrient excess. However, the mechanisms underlying hypothalamic nutrient detection and its impact on peripheral nutrient utilization remain poorly understood. Recent data suggest a role for thioredoxin-interacting protein (TXNIP) as a molecular nutrient sensor important in the regulation of energy metabolism, but the role of hypothalamic TXNIP in the regulation of energy balance has not been evaluated. Here we show in mice that TXNIP is expressed in nutrient-sensing neurons of the mediobasal hypothalamus, responds to hormonal and nutrient signals, and regulates adipose tissue metabolism, fuel partitioning, and glucose homeostasis. Hypothalamic expression of TXNIP is induced by acute nutrient excess and in mouse models of obesity and diabetes, and downregulation of mediobasal hypothalamic TXNIP expression prevents diet-induced obesity and insulin resistance. Thus, mediobasal hypothalamic TXNIP plays a critical role in nutrient sensing and the regulation of fuel utilization.
Figures





References
-
- Andrews ZB, Diano S, Horvath TL. Mitochondrial uncoupling proteins in the CNS: in support of function and survival. Nat Rev Neurosci. 2005;6:829–840. - PubMed
-
- Azzara AV, Sokolnicki JP, Schwartz GJ. Central melanocortin receptor agonist reduces spontaneous and scheduled meal size but does not augment duodenal preload-induced feeding inhibition. Physiol Behav. 2002;77:411–416. - PubMed
-
- Benani A, Troy S, Carmona MC, Fioramonti X, Lorsignol A, Leloup C, Casteilla L, Pénicaud L. Role for mitochondrial reactive oxygen species in brain lipid sensing: redox regulation of food intake. Diabetes. 2007;56:152–160. - PubMed
-
- Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG, Kahn BB. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med. 2006;12:917–924. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
