Orexin gene transfer into zona incerta neurons suppresses muscle paralysis in narcoleptic mice
- PMID: 21508228
- PMCID: PMC3634582
- DOI: 10.1523/JNEUROSCI.6069-10.2011
Orexin gene transfer into zona incerta neurons suppresses muscle paralysis in narcoleptic mice
Abstract
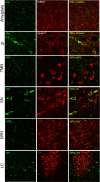
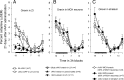
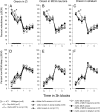
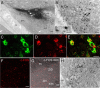
Cataplexy, a sudden unexpected muscle paralysis, is a debilitating symptom of the neurodegenerative sleep disorder, narcolepsy. During these attacks, the person is paralyzed, but fully conscious and aware of their surroundings. To identify potential neurons that might serve as surrogate orexin neurons to suppress such attacks, the gene for orexin (hypocretin), a peptide lost in most human narcoleptics, was delivered into the brains of the orexin-ataxin-3 transgenic mouse model of human narcolepsy. Three weeks after the recombinant adenoassociated virus (rAAV)-mediated orexin gene transfer, sleep-wake behavior was assessed. rAAV-orexin gene delivery into neurons of the zona incerta (ZI), or the lateral hypothalamus (LH) blocked cataplexy. Orexin gene transfer into the striatum or in the melanin-concentrating hormone neurons in the ZI or LH had no such effect, indicating site specificity. In transgenic mice lacking orexin neurons but given rAAV-orexin, detectable levels of orexin-A were evident in the CSF, indicating release of the peptide from the surrogate neurons. Retrograde tracer studies showed that the amygdala innervates the ZI consistent with evidence that strong emotions trigger cataplexy. In turn, the ZI projects to the locus ceruleus, indicating that the ZI is part of a circuit that stabilizes motor tone. Our results indicate that these neurons might also be recruited to block the muscle paralysis in narcolepsy.
Figures






References
-
- Aldrich MS. The neurobiology of narcolepsy-cataplexy. Prog Neurobiol. 1993;41:533–541. - PubMed
-
- Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319:218–245. - PubMed
-
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH055772/MH/NIMH NIH HHS/United States
- R21 HL091363/HL/NHLBI NIH HHS/United States
- R01 NS041454/NS/NINDS NIH HHS/United States
- R01 NS052287/NS/NINDS NIH HHS/United States
- MH055772/MH/NIMH NIH HHS/United States
- NS030140/NS/NINDS NIH HHS/United States
- R01 DK084052/DK/NIDDK NIH HHS/United States
- NS052287/NS/NINDS NIH HHS/United States
- HL091363/HL/NHLBI NIH HHS/United States
- NS41454/NS/NINDS NIH HHS/United States
- R01 NS030140/NS/NINDS NIH HHS/United States
- DK084052/DK/NIDDK NIH HHS/United States
- R01 NS048476/NS/NINDS NIH HHS/United States
- NS48476/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
