Rapid active zone remodeling during synaptic plasticity
- PMID: 21508229
- PMCID: PMC6632979
- DOI: 10.1523/JNEUROSCI.6698-10.2011
Rapid active zone remodeling during synaptic plasticity
Abstract
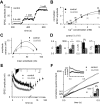
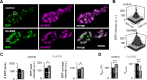
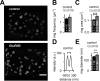
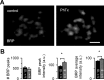
How can synapses change the amount of neurotransmitter released during synaptic plasticity? Although release in general is intensely investigated, its determinants during plasticity are still poorly understood. As a model for plastic strengthening of synaptic release, we here use the well-established presynaptic homeostatic compensation during interference with postsynaptic glutamate receptors at the Drosophila neuromuscular junction. Combining short-term plasticity analysis, cumulative EPSC analysis, fluctuation analysis, and quantal short-term plasticity modeling, we found an increase in the number of release-ready vesicles during presynaptic strengthening. High-resolution light microscopy revealed an increase in the amount of the active zone protein Bruchpilot and an enlargement of the presynaptic cytomatrix structure. Furthermore, these functional and structural alterations of the active zone were not only observed after lifelong but already after minutes of presynaptic strengthening. Our results demonstrate that presynaptic plasticity can induce active zone remodeling, which regulates the number of release-ready vesicles within minutes.
Figures



Similar articles
-
Structural Remodeling of Active Zones Is Associated with Synaptic Homeostasis.J Neurosci. 2020 Apr 1;40(14):2817-2827. doi: 10.1523/JNEUROSCI.2002-19.2020. Epub 2020 Mar 2. J Neurosci. 2020. PMID: 32122953 Free PMC article.
-
Disparate Postsynaptic Induction Mechanisms Ultimately Converge to Drive the Retrograde Enhancement of Presynaptic Efficacy.Cell Rep. 2017 Nov 28;21(9):2339-2347. doi: 10.1016/j.celrep.2017.10.116. Cell Rep. 2017. PMID: 29186673 Free PMC article.
-
Distinct molecular pathways govern presynaptic homeostatic plasticity.Cell Rep. 2021 Dec 14;37(11):110105. doi: 10.1016/j.celrep.2021.110105. Cell Rep. 2021. PMID: 34910905 Free PMC article.
-
Glutamate metabolism, release, and quantal transmission at central excitatory synapses: implications for neural plasticity.Funct Neurol. 1992 Jul-Aug;7(4):315-36. Funct Neurol. 1992. PMID: 1358763 Review.
-
Estimation of quantal parameters at the calyx of Held synapse.Neurosci Res. 2002 Dec;44(4):343-56. doi: 10.1016/s0168-0102(02)00174-8. Neurosci Res. 2002. PMID: 12445623 Review.
Cited by
-
cAMP-Dependent Synaptic Plasticity at the Hippocampal Mossy Fiber Terminal.Front Synaptic Neurosci. 2022 Apr 4;14:861215. doi: 10.3389/fnsyn.2022.861215. eCollection 2022. Front Synaptic Neurosci. 2022. PMID: 35444523 Free PMC article. Review.
-
Drosophila Homolog of the Human Carpenter Syndrome Linked Gene, MEGF8, Is Required for Synapse Development and Function.J Neurosci. 2022 Sep 14;42(37):7016-7030. doi: 10.1523/JNEUROSCI.0442-22.2022. Epub 2022 Aug 9. J Neurosci. 2022. PMID: 35944997 Free PMC article.
-
A Glutamate Homeostat Controls the Presynaptic Inhibition of Neurotransmitter Release.Cell Rep. 2018 May 8;23(6):1716-1727. doi: 10.1016/j.celrep.2018.03.130. Cell Rep. 2018. PMID: 29742428 Free PMC article.
-
α2δ-3 Is Required for Rapid Transsynaptic Homeostatic Signaling.Cell Rep. 2016 Sep 13;16(11):2875-2888. doi: 10.1016/j.celrep.2016.08.030. Cell Rep. 2016. PMID: 27626659 Free PMC article.
-
Bruchpilot and Synaptotagmin collaborate to drive rapid glutamate release and active zone differentiation.Front Cell Neurosci. 2015 Feb 5;9:29. doi: 10.3389/fncel.2015.00029. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25698934 Free PMC article.
References
-
- Albright TD, Jessell TM, Kandel ER, Posner MI. Neural science: a century of progress and the mysteries that remain. Neuron [Suppl] 2000;25:S1–S55. - PubMed
-
- Atwood HL, Wojtowicz JM. Silent synapses in neural plasticity: current evidence. Learn Mem. 1999;6:542–571. - PubMed
-
- Atwood HL, Govind CK, Wu CF. Differential ultrastructure of synaptic terminals on ventral longitudinal abdominal muscles in Drosophila larvae. J Neurobiol. 1993;24:1008–1024. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
