Astrocytes from the contused spinal cord inhibit oligodendrocyte differentiation of adult oligodendrocyte precursor cells by increasing the expression of bone morphogenetic proteins
- PMID: 21508230
- PMCID: PMC3081104
- DOI: 10.1523/JNEUROSCI.5524-09.2011
Astrocytes from the contused spinal cord inhibit oligodendrocyte differentiation of adult oligodendrocyte precursor cells by increasing the expression of bone morphogenetic proteins
Abstract
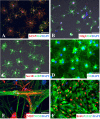
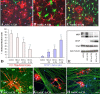
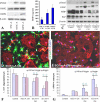
Promotion of remyelination is an important therapeutic strategy to facilitate functional recovery after traumatic spinal cord injury (SCI). Transplantation of neural stem cells (NSCs) or oligodendrocyte precursor cells (OPCs) has been used to enhance remyelination after SCI. However, the microenvironment in the injured spinal cord is inhibitory for oligodendrocyte (OL) differentiation of NSCs or OPCs. Identifying the signaling pathways that inhibit OL differentiation in the injured spinal cord could lead to new therapeutic strategies to enhance remyelination and functional recovery after SCI. In the present study, we show that reactive astrocytes from the injured rat spinal cord or their conditioned media inhibit OL differentiation of adult OPCs with concurrent promotion of astrocyte differentiation. The expression of bone morphogenetic proteins (BMP) is dramatically increased in the reactive astrocytes and their conditioned media. Importantly, blocking BMP activity by BMP receptor antagonist, noggin, reverse the effects of active astrocytes on OPC differentiation by increasing the differentiation of OL from OPCs while decreasing the generation of astrocytes. These data indicate that the upregulated bone morphogenetic proteins in the reactive astrocytes are major factors to inhibit OL differentiation of OPCs and to promote its astrocyte differentiation. These data suggest that manipulation of BMP signaling in the endogenous or grafted NSCs or OPCs may be a useful therapeutic strategy to increase their OL differentiation and remyelination and enhance functional recovery after SCI.
Figures

References
-
- Anderson JM, Hampton DW, Patani R, Pryce G, Crowther RA, Reynolds R, Franklin RJ, Giovannoni G, Compston DA, Baker D, Spillantini MG, Chandran S. Abnormally phosphorylated tau is associated with neuronal and axonal loss in experimental autoimmune encephalomyelitis and multiple sclerosis. Brain. 2008;131:1736–1748. - PubMed
-
- Ara J, See J, Mamontov P, Hahn A, Bannerman P, Pleasure D, Grinspan JB. Bone morphogenetic proteins 4, 6, and 7 are up-regulated in mouse spinal cord during experimental autoimmune encephalomyelitis. J Neurosci Res. 2008;86:125–135. - PubMed
-
- Back SA, Tuohy TM, Chen H, Wallingford N, Craig A, Struve J, Luo NL, Banine F, Liu Y, Chang A, Trapp BD, Bebo BF, Jr, Rao MS, Sherman LS. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005;11:966–972. - PubMed
-
- Blakemore WF, Gilson JM, Crang AJ. The presence of astrocytes in areas of demyelination influences remyelination following transplantation of oligodendrocyte progenitors. Exp Neurol. 2003;184:955–963. - PubMed
-
- Chang A, Tourtellotte WW, Rudick R, Trapp BD. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med. 2002;346:165–173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
