Oligodendrocyte progenitors reversibly exit the cell cycle and give rise to astrocytes in response to interferon-γ
- PMID: 21508246
- PMCID: PMC3104669
- DOI: 10.1523/JNEUROSCI.5905-10.2011
Oligodendrocyte progenitors reversibly exit the cell cycle and give rise to astrocytes in response to interferon-γ
Abstract
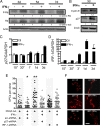
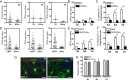
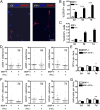
Oligodendrocyte-type 2 astrocyte progenitor cells (O-2A/OPCs) populate the CNS and generate oligodendrocytes and astrocytes in vitro and in vivo. Understanding how O-2A/OPCs respond to their environment is crucial to understanding how these cells function in the CNS and how to best promote their therapeutic proliferation and differentiation. We show that interferon-γ (IFN-γ) was not toxic to highly purified perinatal or adult rat O-2A/OPCs. IFN-γ treatment led to downregulation of PDGFR-α (platelet-derived growth factor receptor-α) and Ki-67 and decreased self-renewal in clonal populations. IFN-γ also significantly increased the proportion of cells in the G(0)/G(1) phase of the cell cycle, decreased BrdU (5-bromo-2'-deoxyuridine) incorporation, and led to increased expression of the cell cycle inhibitors Rb and p27(kip1). Although p27(kip1) expression was not necessary for IFN-γ-mediated quiescence, its upstream regulator IRF-1 was required. The quiescent state of O-2A/OPCs caused by IFN-γ was reversible as the withdrawal of IFN-γ allowed O-2A/OPCs to appropriately respond to both proliferation and differentiation signals. Differentiation into oligodendrocytes induced by either thyroid hormone or CNTF was also abrogated by IFN-γ. This inhibition was specific to the oligodendrocyte pathway, as O-2A/OPC differentiation into astrocytes was not inhibited. IFN-γ alone also led to the generation of GFAP-positive astrocytes in a subset of O-2A/OPCs. Together, these results demonstrate a reversible inhibitory effect of IFN-γ on O-2A/OPC proliferation with a concomitant generation of astrocytes. We propose that neuroinflammation involving increased IFN-γ can reduce progenitor numbers and inhibit differentiation, which has significant clinical relevance for injury repair, but may also contribute to the generation of astrocytes.
Figures





Similar articles
-
Interferon-gamma inhibits cell cycle exit in differentiating oligodendrocyte progenitor cells.Glia. 2005 Nov 1;52(2):127-43. doi: 10.1002/glia.20232. Glia. 2005. PMID: 15920731
-
Oligodendrocyte precursor cells from different brain regions express divergent properties consistent with the differing time courses of myelination in these regions.Dev Biol. 2002 May 15;245(2):362-75. doi: 10.1006/dbio.2002.0610. Dev Biol. 2002. PMID: 11977987
-
Control of division and differentiation in oligodendrocyte-type-2 astrocyte progenitor cells.Ciba Found Symp. 1990;150:227-43; discussion 244-9. doi: 10.1002/9780470513927.ch14. Ciba Found Symp. 1990. PMID: 2373025
-
Redox state as a central modulator of precursor cell function.Ann N Y Acad Sci. 2003 Jun;991:251-71. doi: 10.1111/j.1749-6632.2003.tb07481.x. Ann N Y Acad Sci. 2003. PMID: 12846992 Review.
-
Differentiation of a bipotential glial progenitor cell: what controls the timing and the choice of developmental pathway?J Cell Sci Suppl. 1988;10:77-83. doi: 10.1242/jcs.1988.supplement_10.6. J Cell Sci Suppl. 1988. PMID: 3077944 Review.
Cited by
-
Oligodendroglial heterogeneity in health, disease, and recovery: deeper insights into myelin dynamics.Neural Regen Res. 2025 Nov 1;20(11):3179-3192. doi: 10.4103/NRR.NRR-D-24-00694. Epub 2024 Dec 7. Neural Regen Res. 2025. PMID: 39665821 Free PMC article.
-
Interferon-γ activates nuclear factor-κ B in oligodendrocytes through a process mediated by the unfolded protein response.PLoS One. 2012;7(5):e36408. doi: 10.1371/journal.pone.0036408. Epub 2012 May 4. PLoS One. 2012. PMID: 22574154 Free PMC article.
-
Aberrant Oligodendrogenesis in Down Syndrome: Shift in Gliogenesis?Cells. 2019 Dec 7;8(12):1591. doi: 10.3390/cells8121591. Cells. 2019. PMID: 31817891 Free PMC article. Review.
-
FAM19A5 Expression During Embryogenesis and in the Adult Traumatic Brain of FAM19A5-LacZ Knock-in Mice.Front Neurosci. 2019 Aug 30;13:917. doi: 10.3389/fnins.2019.00917. eCollection 2019. Front Neurosci. 2019. PMID: 31543758 Free PMC article.
-
Transient astrocyte-like NG2 glia subpopulation emerges solely following permanent brain ischemia.Glia. 2021 Nov;69(11):2658-2681. doi: 10.1002/glia.24064. Epub 2021 Jul 27. Glia. 2021. PMID: 34314531 Free PMC article.
References
-
- Agresti C, D'Urso D, Levi G. Reversible inhibitory effects of interferon-gamma and tumour necrosis factor-alpha on oligodendroglial lineage cell proliferation and differentiation in vitro. Eur J Neurosci. 1996;8:1106–1116. - PubMed
-
- Alonso G. NG2 proteoglycan-expressing cells of the adult rat brain: possible involvement in the formation of glial scar astrocytes following stab wound. Glia. 2005;49:318–338. - PubMed
-
- Andrews T, Zhang P, Bhat NR. TNFalpha potentiates IFNgamma-induced cell death in oligodendrocyte progenitors. J Neurosci Res. 1998;54:574–583. - PubMed
-
- Baerwald KD, Popko B. Developing and mature oligodendrocytes respond differently to the immune cytokine interferon-gamma. J Neurosci Res. 1998;52:230–239. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
