The local field potential reflects surplus spike synchrony
- PMID: 21508303
- PMCID: PMC3209854
- DOI: 10.1093/cercor/bhr040
The local field potential reflects surplus spike synchrony
Abstract
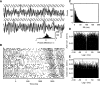
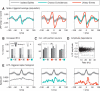
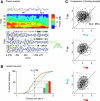
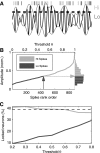
While oscillations of the local field potential (LFP) are commonly attributed to the synchronization of neuronal firing rate on the same time scale, their relationship to coincident spiking in the millisecond range is unknown. Here, we present experimental evidence to reconcile the notions of synchrony at the level of spiking and at the mesoscopic scale. We demonstrate that only in time intervals of significant spike synchrony that cannot be explained on the basis of firing rates, coincident spikes are better phase locked to the LFP than predicted by the locking of the individual spikes. This effect is enhanced in periods of large LFP amplitudes. A quantitative model explains the LFP dynamics by the orchestrated spiking activity in neuronal groups that contribute the observed surplus synchrony. From the correlation analysis, we infer that neurons participate in different constellations but contribute only a fraction of their spikes to temporally precise spike configurations. This finding provides direct evidence for the hypothesized relation that precise spike synchrony constitutes a major temporally and spatially organized component of the LFP.
Figures




Similar articles
-
Dissociation between sustained single-neuron spiking and transient β-LFP oscillations in primate motor cortex.J Neurophysiol. 2017 Apr 1;117(4):1524-1543. doi: 10.1152/jn.00651.2016. Epub 2017 Jan 18. J Neurophysiol. 2017. PMID: 28100654 Free PMC article.
-
Adaptive spike-artifact removal from local field potentials uncovers prominent beta and gamma band neuronal synchronization.J Neurosci Methods. 2020 Jan 15;330:108485. doi: 10.1016/j.jneumeth.2019.108485. Epub 2019 Nov 6. J Neurosci Methods. 2020. PMID: 31705936 Free PMC article.
-
Unbiased and robust quantification of synchronization between spikes and local field potential.J Neurosci Methods. 2016 Aug 30;269:33-8. doi: 10.1016/j.jneumeth.2016.05.004. Epub 2016 May 13. J Neurosci Methods. 2016. PMID: 27180930
-
The asynchronous state's relation to large-scale potentials in cortex.J Neurophysiol. 2019 Dec 1;122(6):2206-2219. doi: 10.1152/jn.00013.2019. Epub 2019 Oct 23. J Neurophysiol. 2019. PMID: 31642401 Free PMC article. Review.
-
Reliability, synchrony and noise.Trends Neurosci. 2008 Aug;31(8):428-34. doi: 10.1016/j.tins.2008.06.002. Epub 2008 Jul 5. Trends Neurosci. 2008. PMID: 18603311 Free PMC article. Review.
Cited by
-
Improved measures of phase-coupling between spikes and the Local Field Potential.J Comput Neurosci. 2012 Aug;33(1):53-75. doi: 10.1007/s10827-011-0374-4. Epub 2011 Dec 21. J Comput Neurosci. 2012. PMID: 22187161 Free PMC article.
-
Hybrid Scheme for Modeling Local Field Potentials from Point-Neuron Networks.Cereb Cortex. 2016 Dec;26(12):4461-4496. doi: 10.1093/cercor/bhw237. Epub 2016 Oct 20. Cereb Cortex. 2016. PMID: 27797828 Free PMC article.
-
VIOLA-A Multi-Purpose and Web-Based Visualization Tool for Neuronal-Network Simulation Output.Front Neuroinform. 2018 Nov 8;12:75. doi: 10.3389/fninf.2018.00075. eCollection 2018. Front Neuroinform. 2018. PMID: 30467469 Free PMC article.
-
Adaptive modeling and inference of higher-order coordination in neuronal assemblies: A dynamic greedy estimation approach.PLoS Comput Biol. 2024 May 28;20(5):e1011605. doi: 10.1371/journal.pcbi.1011605. eCollection 2024 May. PLoS Comput Biol. 2024. PMID: 38805569 Free PMC article.
-
A Single Bout of High-Intensity Cardiovascular Exercise Does Not Enhance Motor Performance and Learning of a Visuomotor Force Modulation Task, but Triggers Ipsilateral Task-Related EEG Activity.Int J Environ Res Public Health. 2021 Nov 27;18(23):12512. doi: 10.3390/ijerph182312512. Int J Environ Res Public Health. 2021. PMID: 34886237 Free PMC article.
References
-
- Aertsen AM, Gerstein GL, Habib MK, Palm G. Dynamics of neuronal firing correlation: modulation of “effective connectivity. J Neurophysiol. 1989;61:900–917. - PubMed
-
- Boashash B. Estimating and interpreting the instantaneous frequency of a signal. I. Fundamentals. Proc IEEE. 1992;80:520–538.
-
- Brown EN, Kass RE, Mitra PP. Multiple neural spike train data analysis: state-of-the-art and future challenges. Nat Neurosci. 2004;7:456–461. - PubMed

