GRB2 couples RhoU to epidermal growth factor receptor signaling and cell migration
- PMID: 21508312
- PMCID: PMC3113775
- DOI: 10.1091/mbc.E10-12-0969
GRB2 couples RhoU to epidermal growth factor receptor signaling and cell migration
Abstract
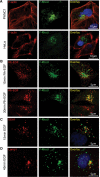
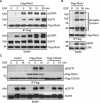
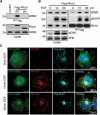
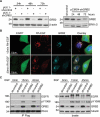
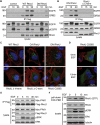
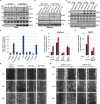
RhoU is an atypical Rho family member with high homology to CDC42 but containing unique N- and C-terminal extensions. The mechanisms regulating RhoU activation, as well as its downstream effectors, are not fully characterized. We show that after epidermal growth factor (EGF) stimulation RhoU colocalizes with EGF receptor (EGFR) on endosomes, which requires both its N- and C-terminal extension sequences. Moreover, RhoU physically associates with activated EGFR in a GRB2-dependent manner through specific proline-rich motifs within its N-terminus. Mutation of these proline-rich sequences or suppression of GRB2 by RNA interference abrogates the interaction of RhoU with activated EGFR, as well as EGF-stimulated RhoU GTP binding. In addition, RhoU is involved in EGFR-mediated signaling, leading to AP1 transcriptional activity and cell migration in pancreatic cancer cells, events that require its interaction with the Grb2-EGFR complex. Taken together, the data suggest a unique regulatory mechanism by which RhoU interaction with SH3 adaptor proteins might serve to integrate growth factor receptor signaling with RhoU activation.
Figures

References
-
- Berzat AC, Buss JE, Chenette EJ, Weinbaum CA, Shutes A, Der CJ, Minden A, Cox AD. Transforming activity of the Rho family GTPase, Wrch-1, a Wnt-regulated Cdc42 homolog, is dependent on a novel carboxyl-terminal palmitoylation motif. J Biol Chem. 2005;280:33055–33065. - PubMed
-
- Brazier H, Pawlak G, Vives V, Blangy A. The Rho GTPase Wrch1 regulates osteoclast precursor adhesion and migration. Int J Biochem Cell Biol. 2009;41:1391–1401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

