Addiction and brain reward and antireward pathways
- PMID: 21508625
- PMCID: PMC4549070
- DOI: 10.1159/000324065
Addiction and brain reward and antireward pathways
Abstract
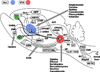
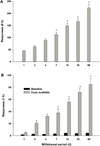
Addictive drugs have in common that they are voluntarily self-administered by laboratory animals (usually avidly), and that they enhance the functioning of the reward circuitry of the brain (producing the 'high' that the drug user seeks). The core reward circuitry consists of an 'in-series' circuit linking the ventral tegmental area, nucleus accumbens and ventral pallidum via the medial forebrain bundle. Although originally believed to simply encode the set point of hedonic tone, these circuits are now believed to be functionally far more complex, also encoding attention, expectancy of reward, disconfirmation of reward expectancy, and incentive motivation. 'Hedonic dysregulation' within these circuits may lead to addiction. The 'second-stage' dopaminergic component in this reward circuitry is the crucial addictive-drug-sensitive component. All addictive drugs have in common that they enhance (directly or indirectly or even transsynaptically) dop-aminergic reward synaptic function in the nucleus accumbens. Drug self-administration is regulated by nucleus accumbens dopamine levels, and is done to keep nucleus accumbens dopamine within a specific elevated range (to maintain a desired hedonic level). For some classes of addictive drugs (e.g. opiates), tolerance to the euphoric effects develops with chronic use. Postuse dysphoria then comes to dominate reward circuit hedonic tone, and addicts no longer use drugs to get high, but simply to get back to normal ('get straight'). The brain circuits mediating the pleasurable effects of addictive drugs are anatomically, neurophysiologically and neurochemically different from those mediating physical dependence, and from those mediating craving and relapse. There are important genetic variations in vulnerability to drug addiction, yet environmental factors such as stress and social defeat also alter brain-reward mechanisms in such a manner as to impart vulnerability to addiction. In short, the 'bio-psycho-social' model of etiology holds very well for addiction. Addiction appears to correlate with a hypodopaminergic dysfunctional state within the reward circuitry of the brain. Neuroimaging studies in humans add credence to this hypothesis. Credible evidence also implicates serotonergic, opioid, endocannabinoid, GABAergic and glutamatergic mechanisms in addiction. Critically, drug addiction progresses from occasional recreational use to impulsive use to habitual compulsive use. This correlates with a progression from reward-driven to habit-driven drug-seeking behavior. This behavioral progression correlates with a neuroanatomical progression from ventral striatal (nucleus accumbens) to dorsal striatal control over drug-seeking behavior. The three classical sets of craving and relapse triggers are (a) reexposure to addictive drugs, (b) stress, and (c) reexposure to environmental cues (people, places, things) previously associated with drug-taking behavior. Drug-triggered relapse involves the nucleus accumbens and the neurotransmitter dopamine. Stress-triggered relapse involves (a) the central nucleus of the amygdala, the bed nucleus of the stria terminalis, and the neurotransmitter corticotrophin-releasing factor, and (b) the lateral tegmental noradrenergic nuclei of the brain stem and the neurotransmitter norepinephrine. Cue-triggered relapse involves the basolateral nucleus of the amygdala, the hippocampus and the neurotransmitter glutamate. Knowledge of the neuroanatomy, neurophysiology, neurochemistry and neuropharmacology of addictive drug action in the brain is currently producing a variety of strategies for pharmacotherapeutic treatment of drug addiction, some of which appear promising.
Copyright © 2011 S. Karger AG, Basel.
Figures






References
-
- Gardner EL. Brain reward mechanisms. In: Lowinson JH, Ruiz P, Millman RB, Langrod JG, editors. Substance Abuse: A Comprehensive Textbook. 4th edn. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 48–97.
-
- Gardner EL. What we have learned about addiction from animal models of drug self-administration. Am J Addict. 2000;9:285–313. - PubMed
-
- O’Brien CP, Gardner EL. Critical assessment of how to study addiction and its treatment: human and non-human animal models. Pharmacol Ther. 2005;108:18–58. - PubMed
-
- Gardner EL, David J. The neurobiology of chemical addiction. In: Elster J, Skog O-J, editors. Getting Hooked: Rationality and the Addictions. Cambridge, England: Cambridge University Press; 1999. pp. 93–136.
-
- Wise RA, Gardner EL. Functional anatomy of substance-related disorders. In: D'haenen H, den Boer JA, Willner P, editors. Biological Psychiatry. New York: Wiley; 2002. pp. 509–522.

