Tacrolimus and sirolimus induce reproductive abnormalities in female rats
- PMID: 21508897
- PMCID: PMC5606232
- DOI: 10.1097/TP.0b013e31821c1e8b
Tacrolimus and sirolimus induce reproductive abnormalities in female rats
Abstract
Background: Immunosuppression medications contribute to posttransplant diabetes mellitus in patients and can cause insulin resistance in male rats. Tacrolimus (TAC)-sirolimus (SIR) immunosuppression is also associated with appearance of ovarian cysts in transplant patients. Because insulin resistance is observed in patients with polycystic ovary syndrome, we hypothesized that TAC or SIR may induce reproductive abnormalities.
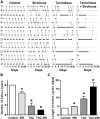
Methods: We monitored estrus cycles of adult female rats treated daily with TAC, SIR, and combination of TAC-SIR, or diluent (control) for 4 weeks. Animals were then challenged with oral glucose to determine their glucose and insulin responses, killed, and their blood and tissues, including ovaries and uteri harvested.
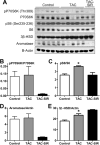
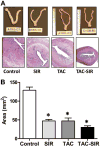
Results: TAC and TAC-SIR treatments increased mean random glucose concentrations (P<0.05). TAC, SIR, and TAC-SIR treatments also increased the glucose response to oral glucose challenge (P<0.05). The insulin response to glucose was significantly higher in rats treated with SIR compared with TAC (P<0.05). TAC, SIR and TAC-SIR treatments reduced number of estrus cycles (P<0.05). The ovaries were smaller after SIR and TAC-SIR treatment compared with controls. The TAC and TAC-SIR treatment groups had fewer preovulatory follicles. Corpora lutea were present in all groups. Ovarian aromatase expression was reduced in the SIR and TAC-SIR treatment groups. A significant (P<0.05) reduction in uterine size was observed in all treatment groups when compared with controls.
Conclusion: In a model of immunosuppressant-induced hyperglycemia, both TAC and SIR induced reproductive abnormalities in adult female rats, likely through different mechanisms.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Davidson J, Wilkinson A, Dantal J, et al. New-onset diabetes after transplantation: 2003 International consensus guidelines. Transplantation; Proceedings of an international expert panel meeting; Barcelona, Spain. 19 February 2003; 2003. p. SS3. - PubMed
-
- Hjelmesaeth J, Hartmann A, Leivestad T, et al. The impact of early-diagnosed new-onset post-transplantation diabetes mellitus on survival and major cardiac events. Kidney Int. 2006;69:588. - PubMed
-
- Sato T, Inagaki A, Uchida K, et al. Diabetes mellitus after transplant: Relationship to pretransplant glucose metabolism and tacrolimus or cyclosporine A-based therapy. Transplantation. 2003;76:1320. - PubMed
-
- Sulanc E, Lane JT, Puumala SE, et al. New-onset diabetes after kidney transplantation: An application of 2003 International Guidelines. Transplantation. 2005;80:945. - PubMed
-
- Penfornis A, Kury-Paulin S. Immunosuppressive drug-induced diabetes. Diabetes Metab. 2006;32(5 pt 2):539. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

