A synthetic homing endonuclease-based gene drive system in the human malaria mosquito
- PMID: 21508956
- PMCID: PMC3093433
- DOI: 10.1038/nature09937
A synthetic homing endonuclease-based gene drive system in the human malaria mosquito
Abstract
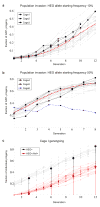
Genetic methods of manipulating or eradicating disease vector populations have long been discussed as an attractive alternative to existing control measures because of their potential advantages in terms of effectiveness and species specificity. The development of genetically engineered malaria-resistant mosquitoes has shown, as a proof of principle, the possibility of targeting the mosquito's ability to serve as a disease vector. The translation of these achievements into control measures requires an effective technology to spread a genetic modification from laboratory mosquitoes to field populations. We have suggested previously that homing endonuclease genes (HEGs), a class of simple selfish genetic elements, could be exploited for this purpose. Here we demonstrate that a synthetic genetic element, consisting of mosquito regulatory regions and the homing endonuclease gene I-SceI, can substantially increase its transmission to the progeny in transgenic mosquitoes of the human malaria vector Anopheles gambiae. We show that the I-SceI element is able to invade receptive mosquito cage populations rapidly, validating mathematical models for the transmission dynamics of HEGs. Molecular analyses confirm that expression of I-SceI in the male germline induces high rates of site-specific chromosomal cleavage and gene conversion, which results in the gain of the I-SceI gene, and underlies the observed genetic drive. These findings demonstrate a new mechanism by which genetic control measures can be implemented. Our results also show in principle how sequence-specific genetic drive elements like HEGs could be used to take the step from the genetic engineering of individuals to the genetic engineering of populations.
Figures

References
-
- Curtis CF. Possible use of translocations to fix desirable genes in insect pest populations. Nature. 1968;218:368–369. - PubMed
-
- Hamilton WD. Extraordinary sex ratios. A sex-ratio theory for sex linkage and inbreeding has new implications in cytogenetics and entomology. Science. 1967;156:477–488. - PubMed
-
- Alphey L, et al. Malaria control with genetically manipulated insect vectors. Science. 2002;298:119–121. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions

