Neuropsin cleaves EphB2 in the amygdala to control anxiety
- PMID: 21508957
- PMCID: PMC3145099
- DOI: 10.1038/nature09938
Neuropsin cleaves EphB2 in the amygdala to control anxiety
Abstract
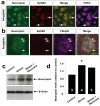
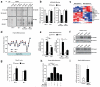
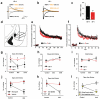
A minority of individuals experiencing traumatic events develop anxiety disorders. The reason for the lack of correspondence between the prevalence of exposure to psychological trauma and the development of anxiety is unknown. Extracellular proteolysis contributes to fear-associated responses by facilitating neuronal plasticity at the neuron-matrix interface. Here we show in mice that the serine protease neuropsin is critical for stress-related plasticity in the amygdala by regulating the dynamics of the EphB2-NMDA-receptor interaction, the expression of Fkbp5 and anxiety-like behaviour. Stress results in neuropsin-dependent cleavage of EphB2 in the amygdala causing dissociation of EphB2 from the NR1 subunit of the NMDA receptor and promoting membrane turnover of EphB2 receptors. Dynamic EphB2-NR1 interaction enhances NMDA receptor current, induces Fkbp5 gene expression and enhances behavioural signatures of anxiety. On stress, neuropsin-deficient mice do not show EphB2 cleavage and its dissociation from NR1 resulting in a static EphB2-NR1 interaction, attenuated induction of the Fkbp5 gene and low anxiety. The behavioural response to stress can be restored by intra-amygdala injection of neuropsin into neuropsin-deficient mice and disrupted by the injection of either anti-EphB2 antibodies or silencing the Fkbp5 gene in the amygdala of wild-type mice. Our findings establish a novel neuronal pathway linking stress-induced proteolysis of EphB2 in the amygdala to anxiety.
Figures

References
Extended Methods’ References
-
- Hirata A, et al. Abnormalities of synapses and neurons in the hippocampus of neuropsin-deficient mice. Mol Cell Neurosci. 2001;17:600–10. - PubMed
-
- Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438:1167–71. - PubMed
-
- Brambilla R, et al. A role for the Ras signalling pathway in synaptic transmission and long-term memory. Nature. 1997;390:281–6. - PubMed
References
-
- Gogolla N, Caroni P, Luthi A, Herry C. Perineuronal nets protect fear memories from erasure. Science. 2009;325:1258–61. - PubMed
-
- Pawlak R, Magarinos AM, Melchor J, McEwen B, Strickland S. Tissue plasminogen activator in the amygdala is critical for stress-induced anxiety-like behavior. Nat Neurosci. 2003;6:168–74. - PubMed
-
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–45. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

