Positive and negative peptide signals control stomatal density
- PMID: 21509541
- PMCID: PMC11114932
- DOI: 10.1007/s00018-011-0685-7
Positive and negative peptide signals control stomatal density
Abstract
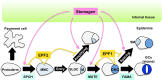
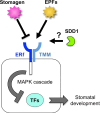
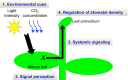
The stoma is a micro valve found on aerial plant organs that promotes gas exchange between the atmosphere and the plant body. Each stoma is formed by a strict cell lineage during the early stages of leaf development. Molecular genetics research using the model plant Arabidopsis has revealed the genes involved in stomatal differentiation. Cysteine-rich secretory peptides of the EPIDERMAL PATTERNING FACTOR-LIKE (EPFL) family play crucial roles as extracellular signaling factors. Stomatal development is orchestrated by the positive factor STOMAGEN/EPFL9 and the negative factors EPF1, EPF2, and CHALLAH/EPFL6 in combination with multiple receptors. EPF1 and EPF2 are produced in the stomatal lineage cells of the epidermis, whereas STOMAGEN and CHALLAH are derived from the inner tissues. These findings highlight the complex cell-to-cell and intertissue communications that regulate stomatal development. To optimize gas exchange, particularly the balance between the uptake of carbon dioxide (CO(2)) and loss of water, plants control stomatal activity in response to environmental conditions. The CO(2) level and light intensity influence stomatal density. Plants sense environmental cues in mature leaves and adjust the stomatal density of newly forming leaves, indicating the involvement of long-distance systemic signaling. This review summarizes recent research progress in the peptide signaling of stomatal development and discusses the evolutionary model of the signaling machinery.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

