Mechanisms inducing low bone density in Duchenne muscular dystrophy in mice and humans
- PMID: 21509823
- PMCID: PMC3150693
- DOI: 10.1002/jbmr.410
Mechanisms inducing low bone density in Duchenne muscular dystrophy in mice and humans
Abstract
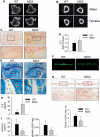
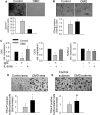
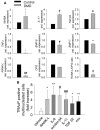
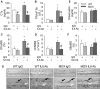
Patients affected by Duchenne muscular dystrophy (DMD) and dystrophic MDX mice were investigated in this study for their bone phenotype and systemic regulators of bone turnover. Micro-computed tomographic (µCT) and histomorphometric analyses showed reduced bone mass and higher osteoclast and bone resorption parameters in MDX mice compared with wild-type mice, whereas osteoblast parameters and mineral apposition rate were lower. In a panel of circulating pro-osteoclastogenic cytokines evaluated in the MDX sera, interleukin 6 (IL-6) was increased compared with wild-type mice. Likewise, DMD patients showed low bone mineral density (BMD) Z-scores and high bone-resorption marker and serum IL-6. Human primary osteoblasts from healthy donors incubated with 10% sera from DMD patients showed decreased nodule mineralization. Many osteogenic genes were downregulated in these cultures, including osterix and osteocalcin, by a mechanism blunted by an IL-6-neutralizing antibody. In contrast, the mRNAs of osteoclastogenic cytokines IL6, IL11, inhibin-βA, and TGFβ2 were increased, although only IL-6 was found to be high in the circulation. Consistently, enhancement of osteoclastogenesis was noted in cultures of circulating mononuclear precursors from DMD patients or from healthy donors cultured in the presence of DMD sera or IL-6. Circulating IL-6 also played a dominant role in osteoclast formation because ex vivo wild-type calvarial bones cultured with 10% sera of MDX mice showed increase osteoclast and bone-resorption parameters that were dampen by treatment with an IL-6 antibody. These results point to IL-6 as an important mediator of bone loss in DMD and suggest that targeted anti-IL-6 therapy may have a positive impact on the bone phenotype in these patients.
Copyright © 2011 American Society for Bone and Mineral Research.
Figures

References
-
- Kunkel LM, Beggs AH, Hoffman EP. Molecular genetics of Duchenne and Becker muscular dystrophy: emphasis on improved diagnosis. Clin Chem. 1989;35:B21–B24. - PubMed
-
- Emery AE. Population frequencies of inherited neuromuscular diseases: a world survey. Neuromusc Disord. 1991;1:19–29. - PubMed
-
- Suresh S, Wales P, Dakin C, Harris MA, Cooper DG. Sleep-related breathing disorder in Duchenne muscular dystrophy: disease spectrum in the paediatric population. J Paediatr Child Health. 2005;41:500–503. - PubMed
-
- Campbell KP. Three muscular dystrophies: loss of cytoskeleton-extracellular matrix linkage. Cell. 1995;80:675–679. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

