NMR-based metabolomic analysis of the molecular pathogenesis of therapy-related myelodysplasia/acute myeloid leukemia
- PMID: 21510650
- PMCID: PMC3390255
- DOI: 10.1021/pr200200y
NMR-based metabolomic analysis of the molecular pathogenesis of therapy-related myelodysplasia/acute myeloid leukemia
Abstract
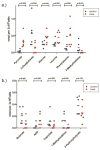
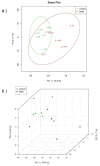
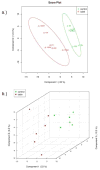
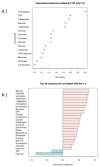
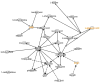
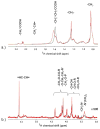
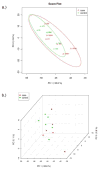
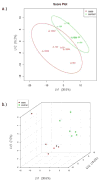
Hematopoietic stem cell transplantation is the oldest and most successful form of stem cell therapy. High dose therapy (HDT) followed by hematopoietic stem cell transplantation allows physicians to administer increased amounts of chemotherapy and/or radiation while minimizing negative side effects such as damage to blood-producing bone marrow cells. Although HDT is successful in treating a wide range of cancers, it leads to lethal therapy-related myelodysplasia syndrome or acute myeloid leukemia (t-MDS/AML) in 5--10% of patients undergoing autologous hematopoietic cell transplantation for Hodgkin lymphoma and non-Hodgkin lymphoma. In this study, we carried out metabolomic analysis of peripheral blood stem cell samples collected in a cohort of patients before hematopoietic cell transplantation to gain insights into the molecular and cellular pathogenesis of t-MDS. Nonparametric tests and multivariate analyses were used to compare the metabolite concentrations in samples from patients that developed t-MDS within 5 years of transplantation and the patients that did not. The results suggest that the development of t-MDS is associated with dysfunctions in cellular metabolic pathways. The top canonical pathways suggested by the metabolomic analysis include alanine and aspartate metabolism, glyoxylate and dicarboxylate metabolism, phenylalanine metabolism, citrate acid cycle, and aminoacyl-t-RNA biosynthesis. Dysfunctions in these pathways indicate mitochondrial dysfunction that would result in decreased ability to detoxify reactive oxygen species generated by chemo and radiation therapy, therefore leading to cancer-causing mutations. These observations suggest predisposing factors for the development of t-MDS.
Figures

References
-
- Hauke RJ, Armitage JO. Treatment of non-Hodgkin lymphoma. Current Opinion in Oncology. 2000;12(5):412–418. - PubMed
-
- Bhatia S, Ramsay NK, Steinbuch M, Dusenbery KE, Shapiro RS, Weisdorf DJ, Robison LL, Miller JS, Neglia JP. Malignant neoplasms following bone marrow transplantation. Blood. 1996;87(9):3633–3639. - PubMed
-
- Miller JS, Arthur DC, Litz CE, Neglia JP, Miller WJ, Weisdorf DJ. Myelodysplastic syndrome after autologous bone marrow transplantation: an additional late complication of curative cancer therapy [see comments] Blood. 1994;83(12):3780–3786. - PubMed
-
- Pedersen-Bjergaard J, Andersen MK, Christiansen DH. Therapy-related acute myeloid leukemia and myelodysplasia after high-dose chemotherapy and autologous stem cell transplantation. Blood. 2000;95(11):3273–3279. - PubMed
-
- Stone RM, Neuberg D, Soiffer R, Takvorian T, Whelan M, Rabinowe SN, Aster JC, Leavitt P, Mauch P, Freedman AS. Myelodysplastic syndrome as a late complication following autologous bone marrow transplantation for non-Hodgkin’s lymphoma. J Clin Oncol. 1994;12(12):2535–2542. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

