Voltage-controlled metal binding on polyelectrolyte-functionalized nanopores
- PMID: 21510657
- PMCID: PMC3099346
- DOI: 10.1021/la2005612
Voltage-controlled metal binding on polyelectrolyte-functionalized nanopores
Abstract
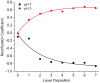
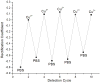
Most of the research in the field of nanopore-based platforms is focused on monitoring ion currents and forces as individual molecules translocate through the nanopore. Molecular gating, however, can occur when target analytes interact with receptors appended to the nanopore surface. Here we show that a solid state nanopore functionalized with polyelectrolytes can reversibly bind metal ions, resulting in a reversible, real-time signal that is concentration dependent. Functionalization of the sensor is based on electrostatic interactions, requires no covalent bond formation, and can be monitored in real time. Furthermore, we demonstrate how the applied voltage can be employed to tune the binding properties of the sensor. The sensor has wide-ranging applications and, its simplest incarnation can be used to study binding thermodynamics using purely electrical measurements with no need for labeling.
Figures





Similar articles
-
Nanoscale Probing of Informational Polymers with Nanopores. Applications to Amyloidogenic Fragments, Peptides, and DNA-PNA Hybrids.Acc Chem Res. 2019 Jan 15;52(1):267-276. doi: 10.1021/acs.accounts.8b00565. Epub 2019 Jan 3. Acc Chem Res. 2019. PMID: 30605305
-
Effect of charge patterns along a solid-state nanopore on polyelectrolyte translocation.J Chem Phys. 2014 Apr 7;140(13):135102. doi: 10.1063/1.4869862. J Chem Phys. 2014. PMID: 24712816 Free PMC article.
-
Ion transport and molecular organization are coupled in polyelectrolyte-modified nanopores.J Am Chem Soc. 2011 Nov 9;133(44):17753-63. doi: 10.1021/ja2063605. Epub 2011 Oct 14. J Am Chem Soc. 2011. PMID: 21942450
-
Plasmonic-Nanopore Biosensors for Superior Single-Molecule Detection.Adv Mater. 2019 Jun;31(23):e1900422. doi: 10.1002/adma.201900422. Epub 2019 Apr 3. Adv Mater. 2019. PMID: 30941823 Review.
-
Biosensing with conically shaped nanopores and nanotubes.Phys Chem Chem Phys. 2006 Nov 21;8(43):4976-88. doi: 10.1039/b607360c. Epub 2006 Aug 7. Phys Chem Chem Phys. 2006. PMID: 17091150 Review.
Cited by
-
Voltage controlled nano-injection system for single-cell surgery.Nanoscale. 2012 Sep 28;4(19):5843-6. doi: 10.1039/c2nr31700a. Epub 2012 Aug 16. Nanoscale. 2012. PMID: 22899383 Free PMC article.
-
The Detection of Trace Metal Contaminants in Organic Products Using Ion Current Rectifying Quartz Nanopipettes.Anal Chem. 2024 Apr 16;96(15):6055-6064. doi: 10.1021/acs.analchem.4c00634. Epub 2024 Apr 3. Anal Chem. 2024. PMID: 38569051 Free PMC article.
-
Rectification of nanopores at surfaces.J Am Chem Soc. 2011 Jul 13;133(27):10398-401. doi: 10.1021/ja203883q. Epub 2011 Jun 21. J Am Chem Soc. 2011. PMID: 21675734 Free PMC article.
-
Nanoneedle-Based Materials for Intracellular Studies.Adv Exp Med Biol. 2021;1295:191-219. doi: 10.1007/978-3-030-58174-9_9. Adv Exp Med Biol. 2021. PMID: 33543461
-
Fundamental studies of nanofluidics: nanopores, nanochannels, and nanopipets.Anal Chem. 2015 Jan 6;87(1):172-87. doi: 10.1021/ac504180h. Epub 2014 Dec 3. Anal Chem. 2015. PMID: 25405581 Free PMC article. Review. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

