Trastuzumab-DM1 causes tumour growth inhibition by mitotic catastrophe in trastuzumab-resistant breast cancer cells in vivo
- PMID: 21510863
- PMCID: PMC3219209
- DOI: 10.1186/bcr2868
Trastuzumab-DM1 causes tumour growth inhibition by mitotic catastrophe in trastuzumab-resistant breast cancer cells in vivo
Abstract
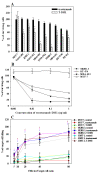
Introduction: Trastuzumab is widely used for the treatment of HER2-positive breast cancer. Despite encouraging clinical results, a significant fraction of patients are, or become, refractory to the drug. To overcome this, trastuzumab-DM1 (T-DM1), a newer, more potent drug has been introduced. We tested the efficacy and mechanisms of action of T-DM1 in nine HER2-positive breast cancer cell lines in vitro and in vivo. The nine cell lines studied included UACC-893, MDA-453 and JIMT-1, which are resistant to both trastuzumab and lapatinib.
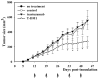
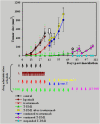
Methods: AlamarBlue cell-proliferation assay was used to determine the growth response of breast cancer cell lines to trastuzumab and T-DM1 in vitro. Trastuzumab- and T-DM1-mediated antibody-dependent cellular cytotoxicity (ADCC) was analysed by measuring the lactate dehydrogenase released from the cancer cells as a result of ADCC activity of peripheral blood mononuclear cells. Severe Combined Immunodeficient (SCID) mice were inoculated with trastuzumab-resistant JIMT-1 cells to investigate the tumour inhibitory effect of T-DM1 in vivo. The xenograft samples were investigated using histology and immunohistochemistry.
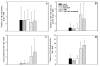
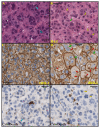
Results: T-DM1 was strongly growth inhibitory on all investigated HER2-positive breast cancer cell lines in vitro. T-DM1 also evoked antibody-dependent cellular cytotoxicity (ADCC) similar to that of trastuzumab. Outgrowth of JIMT-1 xenograft tumours in SCID mice was significantly inhibited by T-DM1. Histologically, the cellular response to T-DM1 consisted of apoptosis and mitotic catastrophe, the latter evidenced by an increased number of cells with aberrant mitotic figures and giant multinucleated cells.
Conclusions: Our results suggest mitotic catastrophe as a previously undescribed mechanism of action of T-DM1. T-DM1 was found effective even on breast cancer cell lines with moderate HER2 expression levels and cross-resistance to trastuzumab and lapatinib (MDA-453 and JIMT-1).
Figures

References
-
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. - DOI - PubMed
-
- Baselga J. Clinical trials of Herceptin(trastuzumab) Eur J Cancer. 2001;37:S18–24. - PubMed
-
- Cuello M, Ettenberg SA, Clark AS, Keane MM, Posner RH, Nau MM, Dennis PA, Lipkowitz S. Down-regulation of the erbB-2 receptor by trastuzumab (herceptin) enhances tumour necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in breast and ovarian cancer cell lines that overexpress erbB-2. Cancer Res. 2001;61:4892–4900. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

