A synergistic antiproliferation effect of curcumin and docosahexaenoic acid in SK-BR-3 breast cancer cells: unique signaling not explained by the effects of either compound alone
- PMID: 21510869
- PMCID: PMC3111403
- DOI: 10.1186/1471-2407-11-149
A synergistic antiproliferation effect of curcumin and docosahexaenoic acid in SK-BR-3 breast cancer cells: unique signaling not explained by the effects of either compound alone
Abstract
Background: Breast cancer is a collection of diseases in which molecular phenotypes can act as both indicators and mediators of therapeutic strategy. Therefore, candidate therapeutics must be assessed in the context of multiple cell lines with known molecular phenotypes. Docosahexaenoic acid (DHA) and curcumin (CCM) are dietary compounds known to antagonize breast cancer cell proliferation. We report that these compounds in combination exert a variable antiproliferative effect across multiple breast cell lines, which is synergistic in SK-BR-3 cells and triggers cell signaling events not predicted by the activity of either compound alone.
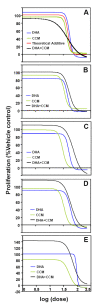
Methods: Dose response curves for CCM and DHA were generated for five breast cell lines. Effects of the DHA+ CCM combination on cell proliferation were evaluated using varying concentrations, at a fixed ratio, of CCM and DHA based on their individual ED₅₀. Detection of synergy was performed using nonlinear regression of a sigmoid dose response model and Combination Index approaches. Cell molecular network responses were investigated through whole genome microarray analysis of transcript level changes. Gene expression results were validated by RT-PCR, and western blot analysis was performed for potential signaling mediators. Cellular curcumin uptake, with and without DHA, was analyzed via flow cytometry and HPLC.
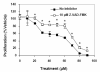
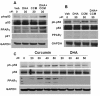
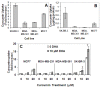
Results: CCM+DHA had an antiproliferative effect in SK-BR-3, MDA-MB-231, MDA-MB-361, MCF7 and MCF10AT cells. The effect was synergistic for SK-BR-3 (ER⁻ PR⁻ Her2⁺) relative to the two compounds individually. A whole genome microarray approach was used to investigate changes in gene expression for the synergistic effects of CCM+DHA in SK-BR-3 cells lines. CCM+DHA triggered transcript-level responses, in disease-relevant functional categories, that were largely non-overlapping with changes caused by CCM or DHA individually. Genes involved in cell cycle arrest, apoptosis, inhibition of metastasis, and cell adhesion were upregulated, whereas genes involved in cancer development and progression, metastasis, and cell cycle progression were downregulated. Cellular pools of PPARγ and phospho-p53 were increased by CCM+DHA relative to either compound alone. DHA enhanced cellular uptake of CCM in SK-BR-3 cells without significantly enhancing CCM uptake in other cell lines.
Conclusions: The combination of DHA and CCM is potentially a dietary supplemental treatment for some breast cancers, likely dependent upon molecular phenotype. DHA enhancement of cellular curcumin uptake is one potential mechanism for observed synergy in SK-BR-3 cells; however, transcriptomic data show that the antiproliferation synergy accompanies many signaling events unique to the combined presence of the two compounds.
Figures


References
-
- Aitken SJ, Thomas JS, Langdon SP, Harrison DJ, Faratian D. Quantitative analysis of changes in ER, PR and HER2 expression in primary breast cancer and paired nodal metastases. Ann Oncol. pp. 1254–1261. - PubMed
-
- Henriksen KL, Rasmussen BB, Lykkesfeldt AE, Moller S, Ejlertsen B, Mouridsen HT. An ER activity profile including ER, PR, Bcl-2 and IGF-IR may have potential as selection criterion for letrozole or tamoxifen treatment of patients with advanced breast cancer. Acta Oncol. 2009;48(4):522–531. doi: 10.1080/02841860802676383. - DOI - PubMed
-
- Lambein K, Praet M, Forsyth R, Van den Broecke R, Braems G, Matthys B, Cocquyt V, Denys H, Pauwels P, Libbrecht L. Relationship between pathological features, HER2 protein expression and HER2 and CEP17 copy number in breast cancer: biological and methodological considerations. J Clin Pathol. pp. 200–207. - PubMed
-
- Parise CA, Bauer KR, Brown MM, Caggiano V. Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999-2004. Breast J. 2009;15(6):593–602. doi: 10.1111/j.1524-4741.2009.00822.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

