Pro-apoptotic activity of α-bisabolol in preclinical models of primary human acute leukemia cells
- PMID: 21510902
- PMCID: PMC3112094
- DOI: 10.1186/1479-5876-9-45
Pro-apoptotic activity of α-bisabolol in preclinical models of primary human acute leukemia cells
Abstract
Background: We previously demonstrated that the plant-derived agent α-bisabolol enters cells via lipid rafts, binds to the pro-apoptotic Bcl-2 family protein BID, and may induce apoptosis. Here we studied the activity of α-bisabolol in acute leukemia cells.
Methods: We tested ex vivo blasts from 42 acute leukemias (14 Philadelphia-negative and 14 Philadelphia-positive B acute lymphoid leukemias, Ph-/Ph+B-ALL; 14 acute myeloid leukemias, AML) for their sensitivity to α-bisabolol in 24-hour dose-response assays. Concentrations and time were chosen based on CD34+, CD33+my and normal peripheral blood cell sensitivity to increasing α-bisabolol concentrations for up to 120 hours.
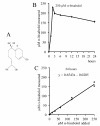
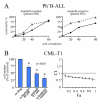
Results: A clustering analysis of the sensitivity over 24 hours identified three clusters. Cluster 1 (14 ± 5 μM α-bisabolol IC50) included mainly Ph-B-ALL cells. AML cells were split into cluster 2 and 3 (45 ± 7 and 65 ± 5 μM IC50). Ph+B-ALL cells were scattered, but mainly grouped into cluster 2. All leukemias, including 3 imatinib-resistant cases, were eventually responsive, but a subset of B-ALL cells was fairly sensitive to low α-bisabolol concentrations. α-bisabolol acted as a pro-apoptotic agent via a direct damage to mitochondrial integrity, which was responsible for the decrease in NADH-supported state 3 respiration and the disruption of the mitochondrial membrane potential.
Conclusion: Our study provides the first evidence that α-bisabolol is a pro-apoptotic agent for primary human acute leukemia cells.
Figures





Similar articles
-
α-bisabolol is an effective proapoptotic agent against BCR-ABL(+) cells in synergism with Imatinib and Nilotinib.PLoS One. 2012;7(10):e46674. doi: 10.1371/journal.pone.0046674. Epub 2012 Oct 3. PLoS One. 2012. PMID: 23056396 Free PMC article.
-
FTY720, a new alternative for treating blast crisis chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphocytic leukemia.J Clin Invest. 2007 Sep;117(9):2408-21. doi: 10.1172/JCI31095. J Clin Invest. 2007. PMID: 17717597 Free PMC article.
-
Enhancement of imatinib-induced apoptosis of BCR/ABL-expressing cells by nutlin-3 through synergistic activation of the mitochondrial apoptotic pathway.Apoptosis. 2010 May;15(5):608-20. doi: 10.1007/s10495-010-0457-0. Apoptosis. 2010. PMID: 20094798
-
The ARF tumor suppressor in acute leukemias: insights from mouse models of Bcr-Abl-induced acute lymphoblastic leukemia.Adv Exp Med Biol. 2007;604:107-14. doi: 10.1007/978-0-387-69116-9_9. Adv Exp Med Biol. 2007. PMID: 17695724 Review.
-
Alpha-bisabolol: unexpected plant-derived weapon in the struggle against tumour survival?Ital J Biochem. 2007 Dec;56(4):323-8. Ital J Biochem. 2007. PMID: 19192636 Review.
Cited by
-
Mitochondrial impairment and oxidative stress mediated apoptosis induced by α-Fe2O3 nanoparticles in Saccharomyces cerevisiae.Toxicol Res (Camb). 2017 Jul 18;6(5):719-728. doi: 10.1039/c7tx00123a. eCollection 2017 Sep 1. Toxicol Res (Camb). 2017. PMID: 30090539 Free PMC article.
-
α-bisabolol enhances radiotherapy-induced apoptosis in endometrial cancer cells by reducing the effect of XIAP on inhibiting caspase-3.Biosci Rep. 2019 Jun 10;39(6):BSR20190696. doi: 10.1042/BSR20190696. Print 2019 Jun 28. Biosci Rep. 2019. Retraction in: Biosci Rep. 2022 Aug 31;42(8):BSR-2019-0696_RET. doi: 10.1042/BSR-2019-0696_RET. PMID: 31127027 Free PMC article. Retracted.
-
Efficient lysis of B-chronic lymphocytic leukemia cells by the plant-derived sesquiterpene alcohol α-bisabolol, a dual proapoptotic and antiautophagic agent.Oncotarget. 2018 May 25;9(40):25877-25890. doi: 10.18632/oncotarget.25398. eCollection 2018 May 25. Oncotarget. 2018. PMID: 29899828 Free PMC article.
-
Pentamidine inhibits prostate cancer progression via selectively inducing mitochondrial DNA depletion and dysfunction.Cell Prolif. 2020 Jan;53(1):e12718. doi: 10.1111/cpr.12718. Epub 2019 Nov 13. Cell Prolif. 2020. PMID: 31721355 Free PMC article.
-
In Vitro Scolicidal Activity of the Sesquiterpenes Isofuranodiene, α-Bisabolol and Farnesol on Echinococcus granulosus Protoscoleces.Molecules. 2020 Aug 7;25(16):3593. doi: 10.3390/molecules25163593. Molecules. 2020. PMID: 32784679 Free PMC article.
References
-
- Costarelli L, Malavolta M, Giacconi R, Cipriano C, Gasparini N, Tesei S, Pierpaoli S, Orlando F, Suzuki H, Perbellini L, Piacenza F, Emanuelli M, Mocchegiani E. In vivo effect of alpha-bisabolol, a nontoxic sesquiterpene alcohol, on the induction of spontaneous mammary tumors in HER-2/neu transgenic mice. Oncol Res. 2010;18:409–418. doi: 10.3727/096504010X12671222663557. - DOI - PubMed
-
- Darra E, Abdel-Azeim S, Manara A, Shoji K, Marechal JD, Mariotto S, Cavalieri E, Perbellini L, Pizza C, Perahia D, Crimi M, Suzuki H. Insight into the apoptosis-inducing action of α-bisabolol towards malignant tumor cells: Involvement of lipid rafts and Bid. Arch Biochem Biophys. 2008;476:113–123. doi: 10.1016/j.abb.2008.02.004. - DOI - PubMed
-
- Appelbaum FR, Rosenblum D, Arceci RJ, Carroll WL, Breitfeld PP, Forman SJ, Larson RA, Lee SJ, Murphy SB, O'Brien S, Radich J, Scher NS, Smith FO, Stone RM, Tallman MS. End points to establish the efficacy of new agents in the treatment of acute leukemia. Blood. 2007;109:1810–1816. doi: 10.1182/blood-2006-08-041152. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

