Early growth response transcription factors: key mediators of fibrosis and novel targets for anti-fibrotic therapy
- PMID: 21511034
- PMCID: PMC3135176
- DOI: 10.1016/j.matbio.2011.03.005
Early growth response transcription factors: key mediators of fibrosis and novel targets for anti-fibrotic therapy
Abstract
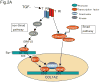
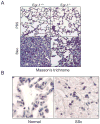
Fibrosis is a deregulated and ultimately defective form of tissue repair that underlies a large number of chronic human diseases, as well as obesity and aging. The pathogenesis of fibrosis involves multiple cell types and extracellular signals, of which transforming growth factor-ß (TGF-ß) is pre-eminent. The prevalence of fibrosis is rising worldwide, and to date no agents has shown clinical efficacy in the attenuating or reversing the process. Recent studies implicate the immediate-early response transcription factor Egr-1 in the pathogenesis of fibrosis. Egr-1 couples acute changes in the cellular environment to sustained alterations in gene expression, and mediates a broad spectrum of biological responses to injury and stress. In contrast to other ligand-activated transcription factors such as NF-κB, c-jun and Smad2/3 that undergo post-translational modification such as phosphorylation and nuclear translocation, Egr-1 activity is regulated via its biosynthesis. Aberrant Egr-1 expression or activity is implicated in cancer, inflammation, atherosclerosis, and ischemic injury and recent studies now indicate an important role for Egr-1 in TGF-ß-dependent profibrotic responses. Fibrosis in various animal models and human diseases such as scleroderma (SSc) and idiopathic pulmonary fibrosis (IPF) is accompanied by aberrant Egr-1 expression. Moreover Egr-1 appears to be required for physiologic and pathological connective tissue remodeling, and Egr-1-null mice are protected from fibrosis. As a novel profibrotic mediator, Egr-1 thus appears to be a promising potential target for the development of anti-fibrotic therapies.
Published by Elsevier B.V.
Figures


References
-
- Bea F, Blessing E, Shelley MI, Shultz JM, Rosenfeld ME. Simvastatin inhibits expression of tissue factor in advanced atherosclerotic lesions of apolipoprotein E deficient mice independently of lipid lowering: potential role of simvastatin-mediated inhibition of Egr-1 expression and activation. Atherosclerosis. 2003;167:187–194. - PubMed
-
- Bhattacharyya S, Chen SJ, Wu M, Blankenship MW, Ning H, Lakos G, Mori Y, Chang E, Nihijima C, Takehara K, Feghali-Bostwick C, Varga J. Smad-Independent Transforming Growth Factor-β Regulation of Early Growth Response-1 and Sustained Expression in Fibrosis. Implications for Scleroderma. Am J Path. 2008;173:1085–1099. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

