Chronic nicotine exposure exacerbates acute renal ischemic injury
- PMID: 21511693
- PMCID: PMC3129886
- DOI: 10.1152/ajprenal.00041.2011
Chronic nicotine exposure exacerbates acute renal ischemic injury
Abstract
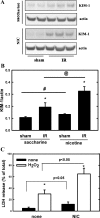
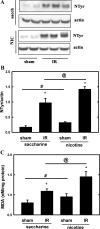
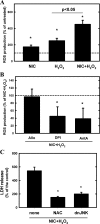
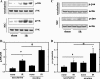
Recent epidemiological reports showed that smoking has a negative impact on renal function and elevates the renal risk not only in the renal patient but perhaps also in the healthy population. Studies suggested that nicotine, a major tobacco alkaloid, links smoking to renal dysfunction. While several studies showed that smoking/chronic nicotine exposure exacerbates the progression of chronic renal diseases, its impact on acute kidney injury is virtually unknown. Here, we studied the effects of chronic nicotine exposure on acute renal ischemic injury. We found that chronic nicotine exposure increased the extent of renal injury induced by warm ischemia-reperfusion as evidenced by morphological changes, increase in plasma creatinine level, and kidney injury molecule-1 expression. We also found that chronic nicotine exposure elevated markers of oxidative stress such as nitrotyrosine as well as malondialdehyde. Interestingly, chronic nicotine exposure alone increased oxidative stress and injury in the kidney without morphological alterations. Chronic nicotine treatment not only increased reactive oxygen species (ROS) production and injury but also exacerbated oxidative stress-induced ROS generation through NADPH oxidase and mitochondria in cultured renal proximal tubule cells. The resultant oxidative stress provoked injury through JNK-mediated activation of the activator protein (AP)-1 transcription factor in vitro. This mechanism might exist in vivo as phosphorylation of JNK and its downstream target c-jun, a component of the AP-1 transcription factor, is elevated in the ischemic kidneys exposed to chronic nicotine. Our results imply that smoking may sensitize the kidney to ischemic insults and perhaps facilitates progression of acute kidney injury to chronic kidney injury.
Figures



References
-
- Arany I, Faisal A, Clark JS, Vera T, Baliga R, Nagamine Y. p66SHC-mediated mitochondrial dysfunction in renal proximal tubule cells during oxidative injury. Am J Physiol Renal Physiol 298: F1214–F1221, 2010 - PubMed
-
- Arany I, Faisal A, Nagamine Y, Safirstein RL. p66shc inhibits pro-survival epidermal growth factor receptor/ERK signaling during severe oxidative stress in mouse renal proximal tubule cells. J Biol Chem 283: 6110–6117, 2008 - PubMed
-
- Arany I, Megyesi JK, Kaneto H, Tanaka S, Safirstein RL. Activation of ERK or inhibition of JNK ameliorates H2O2 cytotoxicity in mouse renal proximal tubule cells. Kidney Int 65: 1231–1239, 2004 - PubMed
-
- Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol 14: 2199–2210, 2003 - PubMed
-
- Briganti EM, Branley P, Chadban SJ, Shaw JE, McNeil JJ, Welborn TA, Atkins RC. Smoking is associated with renal impairment and proteinuria in the normal population: the AusDiab kidney study. Australian Diabetes, Obesity and Lifestyle Study. Am J Kidney Dis 40: 704–712, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

