The G82S polymorphism promotes glycosylation of the receptor for advanced glycation end products (RAGE) at asparagine 81: comparison of wild-type rage with the G82S polymorphic variant
- PMID: 21511948
- PMCID: PMC3122198
- DOI: 10.1074/jbc.M111.241281
The G82S polymorphism promotes glycosylation of the receptor for advanced glycation end products (RAGE) at asparagine 81: comparison of wild-type rage with the G82S polymorphic variant
Abstract
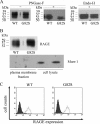
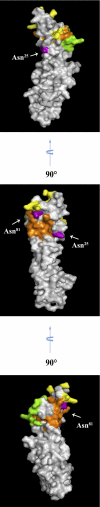
Interaction between the receptor for advanced glycation end products (RAGE) and its ligands amplifies the proinflammatory response. N-Linked glycosylation of RAGE plays an important role in the regulation of ligand binding. Two potential sites for N-linked glycosylation, at Asn(25) and Asn(81), are implicated, one of which is potentially influenced by a naturally occurring polymorphism that substitutes Gly(82) with Ser. This G82S polymorphic RAGE variant displays increased ligand binding and downstream signaling. We hypothesized that the G82S polymorphism affects RAGE glycosylation and thereby affects ligand binding. WT or various mutant forms of RAGE protein, including N25Q, N81Q, N25Q/G82S, and N25Q/N81Q, were produced by transfecting HEK293 cells. The glycosylation patterns of expressed proteins were compared. Enzymatic deglycosylation showed that WT RAGE and the G82S polymorphic variant are glycosylated to the same extent. Our data also revealed N-linked glycosylation of N25Q and N81Q mutants, suggesting that both Asn(25) and Asn(81) can be utilized for N-linked glycosylation. Using mass spectrometry analysis, we found that Asn(81) may or may not be glycosylated in WT RAGE, whereas in G82S RAGE, Asn(81) is always glycosylated. Furthermore, RAGE binding to S100B ligand is affected by Asn(81) glycosylation, with consequences for NF-κB activation. Therefore, the G82S polymorphism promotes N-linked glycosylation of Asn(81), which has implications for the structure of the ligand binding region of RAGE and might explain the enhanced function associated with the G82S polymorphic RAGE variant.
Figures


References
-
- Neeper M., Schmidt A. M., Brett J., Yan S. D., Wang F., Pan Y. C., Elliston K., Stern D., Shaw A. (1992) J. Biol. Chem. 267, 14998–15004 - PubMed
-
- Schmidt A. M., Hori O., Cao R., Yan S. D., Brett J., Wautier J. L., Ogawa S., Kuwabara K., Matsumoto M., Stern D. (1996) Diabetes 45, S77–80 - PubMed
-
- Hofmann M. A., Drury S., Fu C., Qu W., Taguchi A., Lu Y., Avila C., Kambham N., Bierhaus A., Nawroth P., Neurath M. F., Slattery T., Beach D., McClary J., Nagashima M., Morser J., Stern D., Schmidt A. M. (1999) Cell 97, 889–901 - PubMed
-
- Yan S. D., Chen X., Fu J., Chen M., Zhu H., Roher A., Slattery T., Zhao L., Nagashima M., Morser J., Migheli A., Nawroth P., Stern D., Schmidt A. M. (1996) Nature 382, 685–691 - PubMed
-
- Yan S. D., Fu J., Soto C., Chen X., Zhu H., Al-Mohanna F., Collison K., Zhu A., Stern E., Saido T., Tohyama M., Ogawa S., Roher A., Stern D. (1997) Nature 389, 689–695 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

