Spatial coupling of mTOR and autophagy augments secretory phenotypes
- PMID: 21512002
- PMCID: PMC3426290
- DOI: 10.1126/science.1205407
Spatial coupling of mTOR and autophagy augments secretory phenotypes
Abstract
Protein synthesis and autophagic degradation are regulated in an opposite manner by mammalian target of rapamycin (mTOR), whereas under certain conditions it would be beneficial if they occurred in unison to handle rapid protein turnover. We observed a distinct cellular compartment at the trans side of the Golgi apparatus, the TOR-autophagy spatial coupling compartment (TASCC), where (auto)lysosomes and mTOR accumulated during Ras-induced senescence. mTOR recruitment to the TASCC was amino acid- and Rag guanosine triphosphatase-dependent, and disruption of mTOR localization to the TASCC suppressed interleukin-6/8 synthesis. TASCC formation was observed during macrophage differentiation and in glomerular podocytes; both displayed increased protein secretion. The spatial coupling of cells' catabolic and anabolic machinery could augment their respective functions and facilitate the mass synthesis of secretory proteins.
Figures
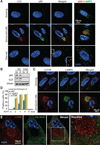
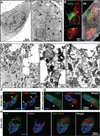


Comment in
-
Cell biology. The TASCC of secretion.Science. 2011 May 20;332(6032):923-5. doi: 10.1126/science.1207552. Science. 2011. PMID: 21596981 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

