Mild elevation of body temperature reduces tumor interstitial fluid pressure and hypoxia and enhances efficacy of radiotherapy in murine tumor models
- PMID: 21512134
- PMCID: PMC3184616
- DOI: 10.1158/0008-5472.CAN-10-4482
Mild elevation of body temperature reduces tumor interstitial fluid pressure and hypoxia and enhances efficacy of radiotherapy in murine tumor models
Abstract
Human and rodent solid tumors often exhibit elevated interstitial fluid pressure (IFP). This condition is recognized as a prognostic indicator for reduced responses to therapy and decreased disease-free survival rate. In the present study, we tested whether induction of a thermoregulatory-mediated increase in tissue blood flow, induced by exposure of mice to mild environmental heat stress, could influence IFP and other vascular parameters within tumors. Using several murine tumor models, we found that heating results in a sustained reduction in tumor IFP correlating with increased tumor vascular perfusion (measured by fluorescent imaging of perfused vessels, laser Doppler flowmetry, and MRI) as well as a sustained reduction in tumor hypoxia. Furthermore, when radiation therapy was administered 24 hours postheating, we observed a significant improvement in efficacy that may be a result of the sustained reduction in tumor hypoxia. These data suggest, for the first time, that environmental manipulation of normal vasomotor function is capable of achieving therapeutically beneficial changes in IFP and microvascular function in the tumor microenvironment.
Figures



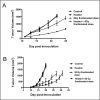
References
-
- Jain RK. Barriers to drug delivery in solid tumors. Sci Am. 1994;271(1):58–65. - PubMed
-
- Vaupel P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin Radiat Oncol. 2004;14(3):198–206. - PubMed
-
- Stohrer M, Boucher Y, Stangassinger M, Jain RK. Oncotic pressure in solid tumors is elevated. Cancer Res. 2000;60(15):4251–5. - PubMed
-
- Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure - An obstacle in cancer therapy. Nature Reviews Cancer. 2004;4(10):806–13. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

