Depletion of Ku70/80 reduces the levels of extrachromosomal telomeric circles and inhibits proliferation of ALT cells
- PMID: 21512205
- PMCID: PMC3117455
- DOI: 10.18632/aging.100308
Depletion of Ku70/80 reduces the levels of extrachromosomal telomeric circles and inhibits proliferation of ALT cells
Abstract
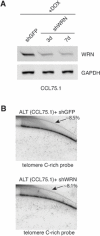
In normal cells, telomeres shorten each time a cell divides ultimately resulting in cell senescence. In contrast, cancer cells counteract the loss of telomeric DNA either by inducing the expression of telomerase or by activating the alternative lengthening of telomeres (ALT) pathway. ALT cells are characterized by heterogeneous telomeres and the presence of extrachromosomal circular double-stranded DNA molecules containing telomeric repeat sequences. These telomeric circles (t-circles) are though to be generated through a recombination process and utilized as templates for telomere elongation by rolling-circle-replication, although their precise mechanism of formation and role in telomere maintenance and cell proliferation is largely unknown. Here we show that shRNA-mediated knockdown of the Ku70/80 heterodimer, a factor with functions at both pathological and natural DNA ends, inhibits ALT cell growth and results in a significant decrease in the levels of t-circles without affecting overall telomere length. These findings demonstrate that non homology-based processes contribute to the maintenance of t-circles and proliferation of ALT cells.
Conflict of interest statement
The authors of this manuscript have no conflict of interests to declare.
Figures



References
-
- Cesare AJ, Reddel RR. Telomere uncapping and alternative lengthening of telomeres. Mech Ageing Dev. 2008;129:99–108. - PubMed
-
- Compton SA, Choi JH, Cesare AJ, Ozgür S, Griffith JD. Xrcc3 and Nbs1 are required for the production of extrachromosomal telomeric circles in human alternative lengthening of telomere cells. Cancer Res. 2007;67:1513–1519. - PubMed
-
- Fisher, Zakian VA. Ku: a multifunctional protein involved in telomere maintenance. DNA Repair (Amst) 2005;4:1215–1226. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

