Immunogenicity is not improved by increased antigen dose or booster dosing of seasonal influenza vaccine in a randomized trial of HIV infected adults
- PMID: 21512577
- PMCID: PMC3064575
- DOI: 10.1371/journal.pone.0017758
Immunogenicity is not improved by increased antigen dose or booster dosing of seasonal influenza vaccine in a randomized trial of HIV infected adults
Abstract
Introduction: The risk of poor vaccine immunogenicity and more severe influenza disease in HIV necessitate strategies to improve vaccine efficacy.
Methods: A randomized, multi-centered, controlled, vaccine trial with three parallel groups was conducted at 12 CIHR Canadian HIV Trials Network sites. Three dosing strategies were used in HIV infected adults (18 to 60 years): two standard doses over 28 days, two double doses over 28 days and a single standard dose of influenza vaccine, administered prior to the 2008 influenza season. A trivalent killed split non-adjuvanted influenza vaccine (Fluviral™) was used. Serum hemagglutinin inhibition (HAI) activity for the three influenza strains in the vaccine was measured to assess immunogenicity.
Results: 297 of 298 participants received at least one injection. Baseline CD4 (median 470 cells/µL) and HIV RNA (76% of patients with viral load <50 copies/mL) were similar between groups. 89% were on HAART. The overall immunogenicity of influenza vaccine across time points and the three influenza strains assessed was poor (Range HAI ≥ 40 = 31-58%). Double dose plus double dose booster slightly increased the proportion achieving HAI titre doubling from baseline for A/Brisbane and B/Florida at weeks 4, 8 and 20 compared to standard vaccine dose. Increased immunogenicity with increased antigen dose and booster dosing was most apparent in participants with unsuppressed HIV RNA at baseline. None of 8 serious adverse events were thought to be immunization-related.
Conclusion: Even with increased antigen dose and booster dosing, non-adjuvanted influenza vaccine immunogenicity is poor in HIV infected individuals. Alternative influenza vaccines are required in this hyporesponsive population.
Trial registration: ClinicalTrials.gov NCT00764998.
Conflict of interest statement
Figures

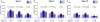
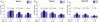

References
-
- Beck JM, Rosen MJ, Peavy HH. Pulmonary complications of HIV infection. 4th NHLBI Workshop: Am J Respir Crit Care Med. 2001:2120–2126. - PubMed
-
- Malaspina A, Moir S, Orsega SM, Vasquez J, Miller NJ, et al. Compromised B cell responses to influenza vaccination in HIV-infected individuals. J Infect Dis. 2005;191:1442–1450. - PubMed
-
- Zanetti AR, Amendola A, Besana S, Boschini A, Tanzi E. Safety and immunogenicity of influenza vaccination in individuals infected with HIV. Vaccine. 2002;20(Suppl 5):B29–32. - PubMed
-
- Colin JF, Cazals-Hatem D, Loriot MA, Martinot-Peignoux M, Pham BN, et al. Influence of human immunodeficiency virus infection on chronic hepatitis B in homosexual men. Hepatology. 1999;29:1306–1310. - PubMed
-
- Cooper CL, Davis HL, Angel JB, Morris ML, Elfer SM, et al. CPG 7909 adjuvant improves hepatitis B virus vaccine seroprotection in antiretroviral-treated HIV-infected adults. AIDS. 2005;19:1473–1479. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

