Mitochondrial structure and function are disrupted by standard isolation methods
- PMID: 21512578
- PMCID: PMC3065478
- DOI: 10.1371/journal.pone.0018317
Mitochondrial structure and function are disrupted by standard isolation methods
Abstract
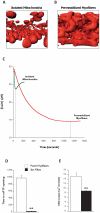
Mitochondria regulate critical components of cellular function via ATP production, reactive oxygen species production, Ca(2+) handling and apoptotic signaling. Two classical methods exist to study mitochondrial function of skeletal muscles: isolated mitochondria and permeabilized myofibers. Whereas mitochondrial isolation removes a portion of the mitochondria from their cellular environment, myofiber permeabilization preserves mitochondrial morphology and functional interactions with other intracellular components. Despite this, isolated mitochondria remain the most commonly used method to infer in vivo mitochondrial function. In this study, we directly compared measures of several key aspects of mitochondrial function in both isolated mitochondria and permeabilized myofibers of rat gastrocnemius muscle. Here we show that mitochondrial isolation i) induced fragmented organelle morphology; ii) dramatically sensitized the permeability transition pore sensitivity to a Ca(2+) challenge; iii) differentially altered mitochondrial respiration depending upon the respiratory conditions; and iv) dramatically increased H(2)O(2) production. These alterations are qualitatively similar to the changes in mitochondrial structure and function observed in vivo after cellular stress-induced mitochondrial fragmentation, but are generally of much greater magnitude. Furthermore, mitochondrial isolation markedly altered electron transport chain protein stoichiometry. Collectively, our results demonstrate that isolated mitochondria possess functional characteristics that differ fundamentally from those of intact mitochondria in permeabilized myofibers. Our work and that of others underscores the importance of studying mitochondrial function in tissue preparations where mitochondrial structure is preserved and all mitochondria are represented.
Conflict of interest statement
Figures

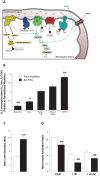
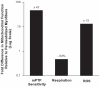
 represent the sequence and site of action of each substrate added to the respirometry assay. Bold italicized items are matrix components which may be partially lost during mitochondrial isolation. (B) Mitochondrial oxygen consumption with sequential substrate addition protocol in both preparations, expressed relative to permeabilized myofibers. Baseline – permeabilized myofibers or isolated mitochondria without substrate; GM – Glutamate-Malate; State 3 GM – GM + ADP; State 3 GMS – State 3 GM + Succinate; TMPD – State 3 GMS + Antimycin A (AA) + TMPD + Ascorbate. (C) Respiratory control ratio (RCR) for both preparations. (D) Mitochondrial respiration ratios calculated for both preparations, representing the relative activity of complexes I, II and IV. Abbreviations: I, II, III, IV, IV – electron transport chain complexes I to IV, and ATP synthase (V); NAD+ – nicotinamide adenine nucleotide; NADH – reduced NAD+; TCA – tricarboxilic acid cycle; MnSOD – manganese superoxide dismutase; GPx – glutathione peroxidase; TPx – thioredoxin peroxidase. N = 8 animals per group, values are means ± s.e.m. * = p<0.05 ** = p<0.01.
represent the sequence and site of action of each substrate added to the respirometry assay. Bold italicized items are matrix components which may be partially lost during mitochondrial isolation. (B) Mitochondrial oxygen consumption with sequential substrate addition protocol in both preparations, expressed relative to permeabilized myofibers. Baseline – permeabilized myofibers or isolated mitochondria without substrate; GM – Glutamate-Malate; State 3 GM – GM + ADP; State 3 GMS – State 3 GM + Succinate; TMPD – State 3 GMS + Antimycin A (AA) + TMPD + Ascorbate. (C) Respiratory control ratio (RCR) for both preparations. (D) Mitochondrial respiration ratios calculated for both preparations, representing the relative activity of complexes I, II and IV. Abbreviations: I, II, III, IV, IV – electron transport chain complexes I to IV, and ATP synthase (V); NAD+ – nicotinamide adenine nucleotide; NADH – reduced NAD+; TCA – tricarboxilic acid cycle; MnSOD – manganese superoxide dismutase; GPx – glutathione peroxidase; TPx – thioredoxin peroxidase. N = 8 animals per group, values are means ± s.e.m. * = p<0.05 ** = p<0.01.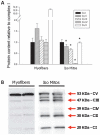

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

