General anesthesia and altered states of arousal: a systems neuroscience analysis
- PMID: 21513454
- PMCID: PMC3390788
- DOI: 10.1146/annurev-neuro-060909-153200
General anesthesia and altered states of arousal: a systems neuroscience analysis
Abstract
Placing a patient in a state of general anesthesia is crucial for safely and humanely performing most surgical and many nonsurgical procedures. How anesthetic drugs create the state of general anesthesia is considered a major mystery of modern medicine. Unconsciousness, induced by altered arousal and/or cognition, is perhaps the most fascinating behavioral state of general anesthesia. We perform a systems neuroscience analysis of the altered arousal states induced by five classes of intravenous anesthetics by relating their behavioral and physiological features to the molecular targets and neural circuits at which these drugs are purported to act. The altered states of arousal are sedation-unconsciousness, sedation-analgesia, dissociative anesthesia, pharmacologic non-REM sleep, and neuroleptic anesthesia. Each altered arousal state results from the anesthetic drugs acting at multiple targets in the central nervous system. Our analysis shows that general anesthesia is less mysterious than currently believed.
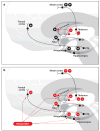
Figures
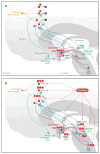
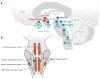
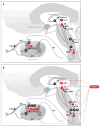

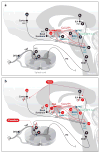
References
-
- Ali HH, Utting JE, Gray TC. Stimulus frequency in the detection of neuromuscular block in humans. Br J Anaesth. 1970;42(11):967–78. - PubMed
-
- Ali HH, Utting JE, Gray TC. Quantitative assessment of residual antidepolarizing block. II. Br J Anaesth. 1971;43(5):478–85. - PubMed
-
- Alkire MT, Haier RJ, Barker SJ, Shah NK, Wu JC, Kao YJ. Cerebral metabolism during propofol anesthesia in humans studied with positron emission tomography. Anesthesiology. 1995;82(2):393–403. discussion 27A. - PubMed
-
- Anand KJ. Anesthetic neurotoxicity in newborns: Should we change clinical practice? Anesthesiology. 2007;107(1):2–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

