Characterization of urine-derived stem cells obtained from upper urinary tract for use in cell-based urological tissue engineering
- PMID: 21513463
- PMCID: PMC9836685
- DOI: 10.1089/ten.TEA.2010.0637
Characterization of urine-derived stem cells obtained from upper urinary tract for use in cell-based urological tissue engineering
Abstract
Background: The goals of this study were to characterize urine-derived stem cells obtained from the upper urinary tract (uUSC), induce these cells to differentiate into urothelial and smooth muscle cells, and determine whether they could serve as a potential stem cell source for bladder tissue engineering.
Materials and methods: Urine samples were collected from five patients with normal upper urinary tracts during renal pyeloplasty. Cells were isolated from this urine and extensively expanded in vitro.
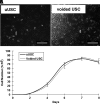
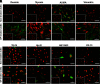
Results: The mean population doubling of uUSC was 46.5±7.7. The uUSC expressed surface markers associated with mesenchymal stem cells and pericytes. These cells could differentiate into smooth muscle-like cells that expressed smooth muscle-specific gene transcripts and proteins, including α-smooth muscle actin, desmin, and myosin, when exposed to TGF-β1 and PDGF-BB. In a collagen lattice assay, these myogenic-differentiated uUSC displayed contractile function that was similar to that seen in native smooth muscle cells. Urothelial-differentiated uUSC expressed urothelial-specific genes and proteins such as uroplakin-Ia and -III, cytokeratin (CK)-7, and CK-13.
Conclusions: uUSC possess expansion and differentiation (urothelial and myogenic) capabilities, and can potentially be used as an alternative cell source in bladder tissue engineering for patients needing cystoplasty.
Conflict of interest statement
No competing financial interests exist.
Figures




References
-
- Atala A. Bauer S.B. Soker S. Yoo J.J. Retik A.B. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241. . - PubMed
-
- Serakinci N. Keith W.N. Therapeutic potential of adult stem cells. Eur J Cancer. 2006;42:1243. . - PubMed
-
- Furth M.E. Atala A. Stem cell sources to treat diabetes. J Cell Biochem. 2009;106:507. . - PubMed
-
- Zhang Y. McNeill E. Tian H. Soker S. Andersson K.E. Yoo J.J. Atala A. Urine derived cells are a potential source for urological tissue reconstruction. J Urol. 2008;180:2226. . - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

