Cerebrospinal fluid hydrodynamics in type I Chiari malformation
- PMID: 21513645
- PMCID: PMC12564500
- DOI: 10.1179/016164111X12962202723805
Cerebrospinal fluid hydrodynamics in type I Chiari malformation
Abstract
Purpose: The objective of this study was to review past studies that have used engineering analysis to examine cerebrospinal fluid hydrodynamics in cranial and spinal subarachnoid spaces in both healthy humans and those affected by type I Chiari malformation.
Methods: A PubMed search of literature pertaining to cerebrospinal fluid hydrodynamics was performed with a particular focus on those that utilized methods such as computational fluid dynamics or experimental flow modeling.
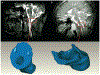
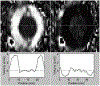
Discussion: From the engineer's perspective, type I Chiari malformation is an abnormal geometry of the cerebellum that causes increased resistance to cerebrospinal fluid flow between the intracranial and spinal subarachnoid space. As such, understanding the hydrodynamics of cerebrospinal fluid in the craniospinal subarachnoid space has long been thought to be important in the diagnosis and management of type I Chiari malformation. Hydrodynamic quantification of cerebrospinal fluid motion in the subarachnoid space may better reflect the pathophysiology of the disorder and serve as a prognostic indicator in conjunction with geometric magnetic resonance measurements that are currently used clinically. This review discusses the results of studies that have sought to quantify the hydrodynamics of cerebrospinal fluid motion using computational and experimental modeling and critiques the methods by which the results were obtained.
Conclusion: Researchers have found differences in cerebrospinal fluid velocities and pressures in type I Chiari malformation patients compared to healthy subjects. However, further research is necessary to determine the causal relationship between changes to hydrodynamic parameters such as cerebrospinal fluid velocity, pressure, resistance to flow, and craniospinal compliance and clinical aspects such as neurological symptoms, radiological evidence of severity, and surgical success.
Figures


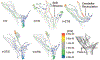

References
-
- Milhorat TH, Chou MW, Trinidad EM, Kula RW, Mandell M, Wolpert C, et al. Chiari I malformation redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurgery 1999;44:1005–17. - PubMed
-
- Elster AD, Chen MY. Chiari I malformations: clinical and radiologic reappraisal. Radiology 1992;183:347–53. - PubMed
-
- Speer MC, Enterline DS, Mehltretter L, Hammock P, Joseph J, Dickerson M, et al. Chiari type I malformation with or without syringomyelia: prevalence and genetics. J Genet Counsel 2003;12:297–311. - PubMed
-
- Speer MC, George TM, Enterline DS, Franklin A, Wolpert CM, Milhorat TH. A genetic hypothesis for Chiari I malformation with or without syringomyelia. Neurosurg Focus 2000;8:E12. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
