Regulation of ciliary motility: conserved protein kinases and phosphatases are targeted and anchored in the ciliary axoneme
- PMID: 21513695
- PMCID: PMC3114296
- DOI: 10.1016/j.abb.2011.04.003
Regulation of ciliary motility: conserved protein kinases and phosphatases are targeted and anchored in the ciliary axoneme
Abstract
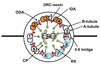
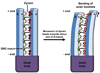
Recent evidence has revealed that the dynein motors and highly conserved signaling proteins are localized within the ciliary 9+2 axoneme. One key mechanism for regulation of motility is phosphorylation. Here, we review diverse evidence, from multiple experimental organisms, that ciliary motility is regulated by phosphorylation/dephosphorylation of the dynein arms through kinases and phosphatases that are anchored immediately adjacent to their axonemal substrates.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



References
-
- Satir P, Christensen ST. Annu Rev Physiol. 2007;69:377–400. - PubMed
-
- Badano JL, Mitsuma N, Beales PL, Katsanis N. Annu Rev Genomics Hum Genet. 2006;7:125–148. - PubMed
-
- Zariwala MA, Knowles MR, Omran H. Annu Rev Physiol. 2007;69:423–450. - PubMed
-
- Sutherland MJ, Ware SM. Am J Med Genet C Semin Med Genet. 2009;151C:307–317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

