Spatiotemporal pattern of neuronal injury induced by DFP in rats: a model for delayed neuronal cell death following acute OP intoxication
- PMID: 21513723
- PMCID: PMC3108263
- DOI: 10.1016/j.taap.2011.03.026
Spatiotemporal pattern of neuronal injury induced by DFP in rats: a model for delayed neuronal cell death following acute OP intoxication
Abstract
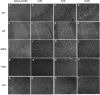
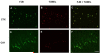
Organophosphate (OP) neurotoxins cause acute cholinergic toxicity and seizures resulting in delayed brain damage and persistent neurological symptoms. Testing novel strategies for protecting against delayed effects of acute OP intoxication has been hampered by the lack of appropriate animal models. In this study, we characterize the spatiotemporal pattern of cellular injury after acute intoxication with the OP diisopropylfluorophosphate (DFP). Adult male Sprague-Dawley rats received pyridostigmine (0.1 mg/kg, im) and atropine methylnitrate (20mg/kg, im) prior to DFP (9 mg/kg, ip) administration. All DFP-treated animals exhibited moderate to severe seizures within minutes after DFP injection but survived up to 72 h. AChE activity was significantly depressed in the cortex, hippocampus, subcortical brain tissue and cerebellum at 1h post-DFP injection and this inhibition persisted for up to 72 h. Analysis of neuronal injury by Fluoro-Jade B (FJB) labeling revealed delayed neuronal cell death in the hippocampus, cortex, amygdala and thalamus, but not the cerebellum, starting at 4h and persisting until 72 h after DFP treatment, although temporal profiles varied between brain regions. At 24h post-DFP injection, the pattern of FJB labeling corresponded to TUNEL staining in most brain regions, and FJB-positive cells displayed reduced NeuN immunoreactivity but were not immunopositive for astrocytic (GFAP), oligodendroglial (O4) or macrophage/microglial (ED1) markers, demonstrating that DFP causes a region-specific delayed neuronal injury mediated in part by apoptosis. These findings indicate the feasibility of this model for testing neuroprotective strategies, and provide insight regarding therapeutic windows for effective pharmacological intervention following acute OP intoxication.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures






References
-
- Allen GV, Gerami D, Esser MJ. Conditioning effects of repetitive mild neurotrauma on motor function in an animal model of focal brain injury. Neuroscience. 2000;99:93–105. - PubMed
-
- Anderson KJ, Fugaccia I, Scheff SW. Fluoro-jade B stains quiescent and reactive astrocytes in the rodent spinal cord. J Neurotrauma. 2003;20:1223–1231. - PubMed
-
- Auta J, Costa E, Davis J, Guidotti A. Imidazenil: a potent and safe protective agent against diisopropyl fluorophosphate toxicity. Neuropharmacology. 2004;46:397–403. - PubMed
-
- Baille V, Clarke PG, Brochier G, Dorandeu F, Verna JM, Four E, Lallement G, Carpentier P. Soman-induced convulsions: the neuropathology revisited. Toxicology. 2005;215:1–24. - PubMed
-
- Baille V, Dorandeu F, Carpentier P, Bizot JC, Filliat P, Four E, Denis J, Lallement G. Acute exposure to a low or mild dose of soman: biochemical, behavioral and histopathological effects. Pharmacology, biochemistry, and behavior. 2001;69:561–569. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

