Mitochondrial dysfunction mediates aldosterone-induced podocyte damage: a therapeutic target of PPARγ
- PMID: 21514419
- PMCID: PMC3081205
- DOI: 10.1016/j.ajpath.2011.01.029
Mitochondrial dysfunction mediates aldosterone-induced podocyte damage: a therapeutic target of PPARγ
Abstract
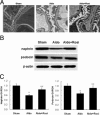
Aldosterone (Aldo) causes podocyte damage by an unknown mechanism. We examined the role of mitochondrial dysfunction (MtD) in Aldo-treated podocytes in vitro and in vivo. Exposure of podocytes to Aldo reduced nephrin expression dose dependently, accompanied by increased production of reactive oxygen species (ROS). The ROS generation and podocyte damage were abolished by the mitochondrial (mt) respiratory chain complex I inhibitor rotenone. Pronounced MtD, including reduced mt membrane potential, ATP levels, and mtDNA copy number were seen in Aldo-treated podocytes and in the glomeruli of Aldo-infused mice. The mineralocorticoid receptor antagonist eplerenone significantly inhibited Aldo-induced MtD. The MtD was associated with higher levels of ROS, reduction in the activity of complexes I, III, and IV, and expression of the peroxisome proliferator-activated receptor-γ (PPARγ) coactivator-1α and mt transcription factor A. Both the PPARγ agonist rosiglitazone and PPARγ overexpression protected against podocyte injury by preventing MtD and oxidative stress, as evidenced by reduced ROS production, by maintenance of mt morphology, by restoration of mtDNA copy number, by decrease in mt membrane potential loss, and by recovery of mt electron transport function. The protective effect of rosiglitazone was abrogated by the specific PPARγ small interference RNA, but not a control small interference RNA. We conclude that MtD is involved in Aldo-induced podocyte injury, and that the PPARγ agonist rosiglitazone may protect podocytes from this injury by improving mitochondrial function.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures











References
-
- Epstein M. Aldosterone as a mediator of progressive renal disease: pathogenetic and clinical implications. Am J Kidney Dis. 2001;37:677–688. - PubMed
-
- Shibata S., Nagase M., Yoshida S., Kawachi H., Fujita T. Podocyte as the target for aldosterone: roles of oxidative stress and Sgk1. Hypertension. 2007;49:355–364. - PubMed
-
- Kiyomoto H., Rafiq K., Mostofa M., Nishiyama A. Possible underlying mechanisms responsible for aldosterone and mineralocorticoid receptor-dependent renal injury. J Pharmacol Sci. 2008;108:399–405. - PubMed
-
- Epstein M. Aldosterone blockade: an emerging strategy for abrogating progressive renal disease. Am J Med. 2006;119:912–919. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

