The early growth response gene Egr2 (Alias Krox20) is a novel transcriptional target of transforming growth factor-β that is up-regulated in systemic sclerosis and mediates profibrotic responses
- PMID: 21514423
- PMCID: PMC3081194
- DOI: 10.1016/j.ajpath.2011.01.035
The early growth response gene Egr2 (Alias Krox20) is a novel transcriptional target of transforming growth factor-β that is up-regulated in systemic sclerosis and mediates profibrotic responses
Erratum in
- Am J Pathol. 2011 Jul;179(1):537. Bhattachyya, Swati [corrected to Bhattacharyya, Swati]
Abstract
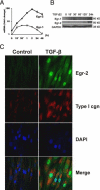
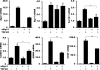
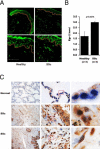
Although the early growth response-2 (Egr-2, alias Krox20) protein shows structural and functional similarities to Egr-1, these two related early-immediate transcription factors are nonredundant. Egr-2 plays essential roles in peripheral nerve myelination, adipogenesis, and immune tolerance; however, its regulation and role in tissue repair and fibrosis remain poorly understood. We show herein that transforming growth factor (TGF)-β induced a Smad3-dependent sustained stimulation of Egr2 gene expression in normal fibroblasts. Overexpression of Egr-2 was sufficient to stimulate collagen gene expression and myofibroblast differentiation, whereas these profibrotic TGF-β responses were attenuated in Egr-2-depleted fibroblasts. Genomewide transcriptional profiling revealed that multiple genes associated with tissue remodeling and wound healing were up-regulated by Egr-2, but the Egr-2-regulated gene expression profile overlapped only partially with the Egr-1-regulated gene profile. Levels of Egr-2 were elevated in lesional tissue from mice with bleomycin-induced scleroderma. Moreover, elevated Egr-2 was noted in biopsy specimens of skin and lung from patients with systemic sclerosis. These results provide the first evidence that Egr-2 is a functionally distinct transcription factor that is both necessary and sufficient for TGF-β-induced profibrotic responses and is aberrantly expressed in lesional tissue in systemic sclerosis and in a murine model of scleroderma. Together, these findings suggest that Egr-2 plays an important nonredundant role in the pathogenesis of fibrosis. Targeting Egr-2 might represent a novel therapeutic strategy to control fibrosis.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
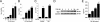


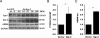



Similar articles
-
Early growth response 3 (Egr-3) is induced by transforming growth factor-β and regulates fibrogenic responses.Am J Pathol. 2013 Oct;183(4):1197-1208. doi: 10.1016/j.ajpath.2013.06.016. Epub 2013 Jul 30. Am J Pathol. 2013. PMID: 23906810 Free PMC article.
-
Smad-independent transforming growth factor-beta regulation of early growth response-1 and sustained expression in fibrosis: implications for scleroderma.Am J Pathol. 2008 Oct;173(4):1085-99. doi: 10.2353/ajpath.2008.080382. Epub 2008 Sep 4. Am J Pathol. 2008. PMID: 18772333 Free PMC article.
-
Egr-1 induces a profibrotic injury/repair gene program associated with systemic sclerosis.PLoS One. 2011;6(9):e23082. doi: 10.1371/journal.pone.0023082. Epub 2011 Sep 13. PLoS One. 2011. PMID: 21931594 Free PMC article.
-
Early growth response transcription factors: key mediators of fibrosis and novel targets for anti-fibrotic therapy.Matrix Biol. 2011 May;30(4):235-42. doi: 10.1016/j.matbio.2011.03.005. Epub 2011 Apr 13. Matrix Biol. 2011. PMID: 21511034 Free PMC article. Review.
-
Transcriptional profiling of the scleroderma fibroblast reveals a potential role for connective tissue growth factor (CTGF) in pathological fibrosis.Keio J Med. 2004 Jun;53(2):74-7. doi: 10.2302/kjm.53.74. Keio J Med. 2004. PMID: 15247510 Review.
Cited by
-
Role of Areca Nut Induced TGF-β and Epithelial-Mesenchymal Interaction in the Pathogenesis of Oral Submucous Fibrosis.PLoS One. 2015 Jun 24;10(6):e0129252. doi: 10.1371/journal.pone.0129252. eCollection 2015. PLoS One. 2015. PMID: 26107172 Free PMC article.
-
Toll-like receptor 4 signaling augments transforming growth factor-β responses: a novel mechanism for maintaining and amplifying fibrosis in scleroderma.Am J Pathol. 2013 Jan;182(1):192-205. doi: 10.1016/j.ajpath.2012.09.007. Epub 2012 Nov 7. Am J Pathol. 2013. PMID: 23141927 Free PMC article.
-
Streptolysin S induces pronounced calcium-ion influx-dependent expression of immediate early genes encoding transcription factors.Sci Rep. 2023 Aug 22;13(1):13720. doi: 10.1038/s41598-023-40981-1. Sci Rep. 2023. PMID: 37608082 Free PMC article.
-
Egr2 induction in spiny projection neurons of the ventrolateral striatum contributes to cocaine place preference in mice.Elife. 2021 Mar 16;10:e65228. doi: 10.7554/eLife.65228. Elife. 2021. PMID: 33724178 Free PMC article.
-
Accelerated ageing and coronary microvascular dysfunction in chronic heart failure in Tgαq*44 mice.Geroscience. 2023 Jun;45(3):1619-1648. doi: 10.1007/s11357-022-00716-y. Epub 2023 Jan 24. Geroscience. 2023. PMID: 36692592 Free PMC article.
References
-
- Jimenez S.A., Derk C.T. Following the molecular pathways toward an understanding of the pathogenesis of systemic sclerosis. Ann Intern Med. 2004;140:37–50. - PubMed
-
- Gabrielli A., Avvedimento E.V., Krieg T. Scleroderma. N Engl J Med. 2009;360:1989–2003. - PubMed
-
- Abraham D.J., Varga J. Scleroderma: from cell and molecular mechanisms to disease models. Trends Immunol. 2005;26:587–595. - PubMed
-
- Heldin C.H., Landstrom M., Moustakas A. Mechanism of TGF-beta signaling to growth arrest, apoptosis, and epithelial-mesenchymal transition. Curr Opin Cell Biol. 2009;21:166–176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

