Connexin37 and Connexin43 deficiencies in mice disrupt lymphatic valve development and result in lymphatic disorders including lymphedema and chylothorax
- PMID: 21515254
- PMCID: PMC3134316
- DOI: 10.1016/j.ydbio.2011.04.004
Connexin37 and Connexin43 deficiencies in mice disrupt lymphatic valve development and result in lymphatic disorders including lymphedema and chylothorax
Abstract
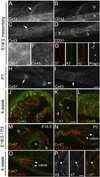
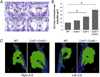
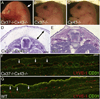
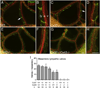
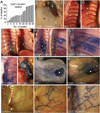
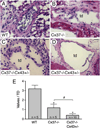
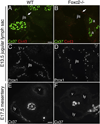
Intraluminal valves are required for the proper function of lymphatic collecting vessels and large lymphatic trunks like the thoracic duct. Despite recent progress in the study of lymphvasculogenesis and lymphangiogenesis, the molecular mechanisms controlling the morphogenesis of lymphatic valves remain poorly understood. Here, we report that gap junction proteins, or connexins (Cxs), are required for lymphatic valvulogenesis. Cx37 and Cx43 are expressed early in mouse lymphatic development in the jugular lymph sacs, and later in development these Cxs become enriched and differentially expressed by lymphatic endothelial cells on the upstream and downstream sides of the valves. Specific deficiencies of Cx37 and Cx43 alone or in combination result in defective valve formation in lymphatic collecting vessels, lymphedema, and chylothorax. We also show that Cx37 regulates jugular lymph sac size and that both Cx37 and Cx43 are required for normal thoracic duct development, including valve formation. Another Cx family member, Cx47, whose human analog is mutated in some families with lymphedema, is also highly enriched in a subset of endothelial cells in lymphatic valves. Mechanistically, we present data from Foxc2-/- embryos suggesting that Cx37 may be a target of regulation by Foxc2, a transcription factor that is mutated in human lymphedema-distichiasis syndrome. These results show that at least three Cxs are expressed in the developing lymphatic vasculature and, when defective, are associated with clinically manifest lymphatic disorders in mice and man.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures




References
-
- Bannykh S, Mironov A, Bannykh G, Mironov A. The morphology of valves and valve-like structures in the canine and feline thoracic duct. Anat. Embryol. (Berl) 1995;192:265–274. - PubMed
-
- Bertozzi CC, Schmaier AA, Mericko P, Hess PR, Zou Z, Chen M, Chen CY, Xu B, Lu MM, Zhou D, Sebzda E, Santore MT, Merianos DJ, Stadtfeld M, Flake AW, Graf T, Skoda R, Maltzman JS, Koretzky GA, Kahn ML. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood. 2010;116:661–670. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

