Quantitative proteomics analysis of phosphorylated proteins in the hippocampus of Alzheimer's disease subjects
- PMID: 21515431
- PMCID: PMC3119855
- DOI: 10.1016/j.jprot.2011.03.033
Quantitative proteomics analysis of phosphorylated proteins in the hippocampus of Alzheimer's disease subjects
Abstract
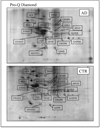
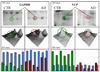
Phosphorylation on tyrosine, threonine and serine residues represents one of the most important post-translational modifications and is a key regulator of cellular signaling of multiple biological processes that require a strict control by protein kinases and protein phosphatases. Abnormal protein phosphorylation has been associated with several human diseases including Alzheimer's disease (AD). One of the characteristic hallmarks of AD is the presence of neurofibrillary tangles, composed of microtubule-associated, abnormally hyperphosphorylated tau protein. However, several others proteins showed altered phosphorylation levels in AD suggesting that deregulated phosphorylation may contribute to AD pathogenesis. Phosphoproteomics has recently gained attention as a valuable approach to analyze protein phosphorylation, both in a quantitative and a qualitative way. We used the fluorescent phosphospecific Pro-Q Diamond dye to identify proteins that showed alterations in their overall phosphorylation in the hippocampus of AD vs. control (CTR) subjects. Significant changes were found for 17 proteins involved in crucial neuronal process such as energy metabolism or signal transduction. These phosphoproteome data may provide new clues to better understand molecular pathways that are deregulated in the pathogenesis and progression of AD.
Copyright © 2011 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no financial/commercial conflicts of interests with the results of this study.
Figures

References
-
- Walsh CT. Posttranslational modification of proteins: expanding nature's inventory. Greenwood Village, CO: Roberts and Company Publishers; 2006.
-
- Zhou H, Elisma F, Denis NJ, Wright TG, Tian R, Hou W, et al. Analysis of the subcellular phosphoproteome using a novel phosphoproteomic reactor. J Proteome Res. 2010;9:1279–1288. - PubMed
-
- Cohen P. The origins of protein phosphorylation. Nat Cell Biol. 2002;4:E127–E130. - PubMed
-
- Ruan L, Wang GL, Chen Y, Yi H, Tang CE, Zhang PF, et al. Identification of tyrosine phosphoproteins in signaling pathway triggered TGF-a by using functional proteomics technology. Med Oncol. 2010 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

