Coexpressed D1- and D2-like dopamine receptors antagonistically modulate acetylcholine release in Caenorhabditis elegans
- PMID: 21515580
- PMCID: PMC3176529
- DOI: 10.1534/genetics.111.128512
Coexpressed D1- and D2-like dopamine receptors antagonistically modulate acetylcholine release in Caenorhabditis elegans
Abstract
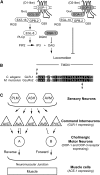
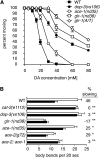
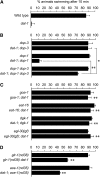
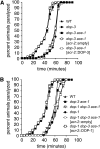
Dopamine acts through two classes of G protein-coupled receptor (D1-like and D2-like) to modulate neuron activity in the brain. While subtypes of D1- and D2-like receptors are coexpressed in many neurons of the mammalian brain, it is unclear how signaling by these coexpressed receptors interacts to modulate the activity of the neuron in which they are expressed. D1- and D2-like dopamine receptors are also coexpressed in the cholinergic ventral-cord motor neurons of Caenorhabditis elegans. To begin to understand how coexpressed dopamine receptors interact to modulate neuron activity, we performed a genetic screen in C. elegans and isolated mutants defective in dopamine response. These mutants were also defective in behaviors mediated by endogenous dopamine signaling, including basal slowing and swimming-induced paralysis. We used transgene rescue experiments to show that defects in these dopamine-specific behaviors were caused by abnormal signaling in the cholinergic motor neurons. To investigate the interaction between the D1- and D2-like receptors specifically in these cholinergic motor neurons, we measured the sensitivity of dopamine-signaling mutants and transgenic animals to the acetylcholinesterase inhibitor aldicarb. We found that D2 signaling inhibited acetylcholine release from the cholinergic motor neurons while D1 signaling stimulated release from these same cells. Thus, coexpressed D1- and D2-like dopamine receptors act antagonistically in vivo to modulate acetylcholine release from the cholinergic motor neurons of C. elegans.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

