Dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin (DC-SIGN) recognizes a novel ligand, Mac-2-binding protein, characteristically expressed on human colorectal carcinomas
- PMID: 21515679
- PMCID: PMC3121387
- DOI: 10.1074/jbc.M110.215301
Dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin (DC-SIGN) recognizes a novel ligand, Mac-2-binding protein, characteristically expressed on human colorectal carcinomas
Abstract
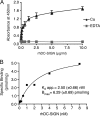
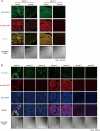
Dendritic cell (DC)-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) is a type II transmembrane C-type lectin expressed on DCs such as myeloid DCs and monocyte-derived DCs (MoDCs). Recently, we have reported that DC-SIGN interacts with carcinoembryonic antigen (CEA) expressed on colorectal carcinoma cells. CEA is one of the most widely used tumor markers for gastrointestinal cancers such as colorectal cancer. On the other hand, other groups have reported that the level of Mac-2-binding protein (Mac-2BP) increases in patients with pancreatic, breast, and lung cancers, virus infections such as human immunodeficiency virus and hepatitis C virus, and autoimmune diseases. Here, we first identified Mac-2BP expressed on several colorectal carcinoma cell lines as a novel DC-SIGN ligand through affinity chromatography and mass spectrometry. Interestingly, we found that DC-SIGN selectively recognizes Mac-2BP derived from some colorectal carcinomas but not from the other ones. Furthermore, we found that the α1-3,4-fucose moieties of Le glycans expressed on DC-SIGN-binding Mac-2BP were important for recognition. DC-SIGN-dependent cellular interactions between immature MoDCs and colorectal carcinoma cells significantly inhibited MoDC functional maturation, suggesting that Mac-2BP may provide a tolerogenic microenvironment for colorectal carcinoma cells through DC-SIGN-dependent recognition. Importantly, Mac-2BP was detected as a predominant DC-SIGN ligand expressed on some primary colorectal cancer tissues from certain parts of patients in comparison with CEA from other parts, suggesting that DC-SIGN-binding Mac-2BP bearing tumor-associated Le glycans may become a novel potential colorectal cancer biomarker for some patients instead of CEA.
Figures





References
-
- Banchereau J., Briere F., Caux C., Davoust J., Lebecque S., Liu Y. J., Pulendran B., Palucka K. (2000) Annu. Rev. Immunol. 18, 767–811 - PubMed
-
- Lanzavecchia A., Sallusto F. (2001) Cell 106, 263–266 - PubMed
-
- Geijtenbeek T. B., van Vliet S. J., Engering A., ‘t Hart B. A., van Kooyk Y. (2004) Annu. Rev. Immunol. 22, 33–54 - PubMed
-
- van Vliet S. J., den Dunnen J., Gringhuis S. I., Geijtenbeek T. B., van Kooyk Y. (2007) Curr. Opin. Immunol. 19, 435–440 - PubMed
-
- van Kooyk Y., Rabinovich G. A. (2008) Nat. Immunol. 9, 593–601 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

