Lettuce costunolide synthase (CYP71BL2) and its homolog (CYP71BL1) from sunflower catalyze distinct regio- and stereoselective hydroxylations in sesquiterpene lactone metabolism
- PMID: 21515683
- PMCID: PMC3122218
- DOI: 10.1074/jbc.M110.216804
Lettuce costunolide synthase (CYP71BL2) and its homolog (CYP71BL1) from sunflower catalyze distinct regio- and stereoselective hydroxylations in sesquiterpene lactone metabolism
Abstract
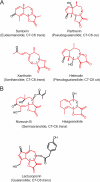
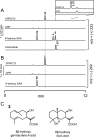
Sesquiterpene lactones (STLs) are terpenoid natural products possessing the γ-lactone, well known for their diverse biological and medicinal activities. The occurrence of STLs is sporadic in nature, but most STLs have been isolated from plants in the Asteraceae family. Despite the implication of the γ-lactone group in many reported bioactivities of STLs, the biosynthetic origins of the γ-lactone ring remains elusive. Germacrene A acid (GAA) has been suggested as a central precursor of diverse STLs. The regioselective (C6 or C8) and stereoselective (α or β) hydroxylation on a carbon of GAA adjacent to its carboxylic acid at C12 is responsible for the γ-lactone formation. Here, we report two cytochrome P450 monooxygenases (P450s) capable of catalyzing 6α- and 8β-hydroxylation of GAA from lettuce and sunflower, respectively. To identify these P450s, sunflower trichomes were isolated to generate a trichome-specific transcript library, from which 10 P450 clones were retrieved. Expression of these clones in a yeast strain metabolically engineered to synthesize substrate GAA identified a P450 catalyzing 8β-hydroxylation of GAA, but the STL was not formed by spontaneous lactonization. Subsequently, we identified the closest homolog of the GAA 8β-hydroxylase from lettuce and discovered 6α-hydroxylation of GAA by the recombinant enzyme. The resulting 6α-hydroxy-GAA spontaneously undergoes a lactonization to yield the simplest form of STL, costunolide. Furthermore, we demonstrate the milligram per liter scale de novo synthesis of costunolide using the lettuce P450 in an engineered yeast strain, an important advance that will enable exploitation of STLs. Evolution and homology models of these two P450s are discussed.
Figures
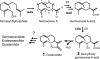
References
-
- Eckstein-Ludwig U., Webb R. J., Van Goethem I. D., East J. M., Lee A. G., Kimura M., O'Neill P. M., Bray P. G., Ward S. A., Krishna S. (2003) Nature 424, 957–961 - PubMed
-
- Kwok B. H., Koh B., Ndubuisi M. I., Elofsson M., Crews C. M. (2001) Chem. Biol. 8, 759–766 - PubMed
-
- Satou T., Koga M., Matsuhashi R., Koike K., Tada I., Nikaido T. (2002) Vet. Parasitol. 104, 131–138 - PubMed
-
- Christensen S. B., Andersen A., Kromann H., Treiman M., Tombal B., Denmeade S., Isaacs J. T. (1999) Bioorg. Med. Chem. 7, 1273–1280 - PubMed
-
- Picman A. K. (1986) Biochem. Syst. Ecol. 14, 255–281
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

