Incidence of diabetes following ramipril or rosiglitazone withdrawal
- PMID: 21515846
- PMCID: PMC3114353
- DOI: 10.2337/dc10-1567
Incidence of diabetes following ramipril or rosiglitazone withdrawal
Abstract
Objective: To examine the impact of withdrawing rosiglitazone and ramipril medication on diabetes incidence after closeout of the Diabetes REduction Assessment with ramipril and rosiglitazone Medication (DREAM) trial.
Research design and methods: The 3,366 DREAM subjects at trial end who had not developed diabetes while taking double-blind study medication were transferred to single-blind placebo for 2 to 3 months before undergoing an oral glucose tolerance test. Glycemic status was analyzed for the trial plus washout period and for the washout period alone.
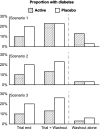
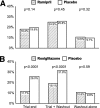
Results: Following median (interquartile range) 71 (63-86) days drug withdrawal, overall glycemic status remained modestly improved in those allocated ramipril during the trial with an 11% increase in regression to normoglycemia, compared with placebo. In those previously allocated rosiglitazone, glycemic status remained substantially improved with a 49% reduction of new-onset diabetes or death and a 22% increase in regression to normoglycemia, compared with placebo. However, during the washout phase alone the incidence of diabetes or death was identical for those allocated previously to ramipril or placebo, or to rosiglitazone or placebo.
Conclusions: In people allocated to ramipril compared with those not allocated ramipril during the trial, the postwashout normoglycemia incidence was higher. In people allocated to rosiglitazone compared with those not allocated rosiglitazone during the trial, the postwashout incidence of diabetes was significantly lower and the incidence of normoglycemia was higher. During the washout period, diabetes incidence was the same for ramipril versus placebo and for rosiglitazone versus placebo. Rosiglitazone delays disease progression during treatment but the process resumes at the placebo rate when the drug is stopped.
Trial registration: ClinicalTrials.gov NCT00095654.
Figures
Similar articles
-
[Influence of diabetes incidence by ramipril and rosiglitazone: DREAM study (diabetes reduction assessment with ramipril and rosiglitazone medication)].Internist (Berl). 2007 Oct;48(10):1173-6. doi: 10.1007/s00108-007-1932-8. Internist (Berl). 2007. PMID: 17846732 German. No abstract available.
-
[DREAM study: prevention of type 2 diabetes with ramipril and/or rosiglitazone in persons with dysglycaemia but no cardiovascular desease].Rev Med Liege. 2006 Oct;61(10):728-32. Rev Med Liege. 2006. PMID: 17209507 Clinical Trial. French.
-
Effects of ramipril and rosiglitazone on cardiovascular and renal outcomes in people with impaired glucose tolerance or impaired fasting glucose: results of the Diabetes REduction Assessment with ramipril and rosiglitazone Medication (DREAM) trial.Diabetes Care. 2008 May;31(5):1007-14. doi: 10.2337/dc07-1868. Epub 2008 Feb 11. Diabetes Care. 2008. PMID: 18268075 Clinical Trial.
-
[Experiences of the DREAM trial].Orv Hetil. 2006 Dec 31;147(52):2523-6. Orv Hetil. 2006. PMID: 17294577 Review. Hungarian.
-
Repaglinide : a pharmacoeconomic review of its use in type 2 diabetes mellitus.Pharmacoeconomics. 2004;22(6):389-411. doi: 10.2165/00019053-200422060-00005. Pharmacoeconomics. 2004. PMID: 15099124 Review.
Cited by
-
Treatment of prediabetes.World J Diabetes. 2015 Sep 25;6(12):1207-22. doi: 10.4239/wjd.v6.i12.1207. World J Diabetes. 2015. PMID: 26464759 Free PMC article. Review.
-
Preventing diabetes mellitus in developing countries.Nat Rev Endocrinol. 2012 Sep;8(9):557-62. doi: 10.1038/nrendo.2012.46. Epub 2012 Apr 10. Nat Rev Endocrinol. 2012. PMID: 22488646 Review.
-
Pioglitazone Prevents Diabetes in Patients With Insulin Resistance and Cerebrovascular Disease.Diabetes Care. 2016 Oct;39(10):1684-92. doi: 10.2337/dc16-0798. Epub 2016 Jul 27. Diabetes Care. 2016. PMID: 27465265 Free PMC article. Clinical Trial.
-
Should Prediabetes be Treated Pharmacologically?Diabetes Ther. 2023 Oct;14(10):1585-1593. doi: 10.1007/s13300-023-01449-7. Epub 2023 Jul 25. Diabetes Ther. 2023. PMID: 37490238 Free PMC article.
-
Standards of medical care in diabetes--2012.Diabetes Care. 2012 Jan;35 Suppl 1(Suppl 1):S11-63. doi: 10.2337/dc12-s011. Diabetes Care. 2012. PMID: 22187469 Free PMC article. Review. No abstract available.
References
-
- Gerstein HC, Yusuf S, Holman RR, Bosch J, Pogue J; DREAM Trial Investigators Rationale, design and recruitment characteristics of a large, simple international trial of diabetes prevention: the DREAM trial. Diabetologia 2004;47:1519–1527 - PubMed
-
- Gerstein HC, Yusuf S, Bosch J, et al. ; DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 2006;368:1096–1105 - PubMed
-
- Bosch J, Yusuf S, Gerstein HC, et al. ; DREAM Trial Investigators Effect of ramipril on the incidence of diabetes. N Engl J Med 2006;355:1551–1562 - PubMed
-
- Buchanan TA, Xiang AH, Peters RK, et al. Preservation of pancreatic β-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes 2002;51:2796–2803 - PubMed

