Biomechanics of leukocyte rolling
- PMID: 21515934
- PMCID: PMC3103268
- DOI: 10.3233/BIR-2011-0579
Biomechanics of leukocyte rolling
Abstract
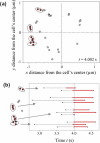
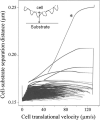
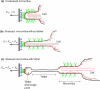
Leukocyte rolling on endothelial cells and other P-selectin substrates is mediated by P-selectin binding to P-selectin glycoprotein ligand-1 expressed on the tips of leukocyte microvilli. Leukocyte rolling is a result of rapid, yet balanced formation and dissociation of selectin-ligand bonds in the presence of hydrodynamic shear forces. The hydrodynamic forces acting on the bonds may either increase (catch bonds) or decrease (slip bonds) their lifetimes. The force-dependent 'catch-slip' bond kinetics are explained using the 'two pathway model' for bond dissociation. Both the 'sliding-rebinding' and the 'allosteric' mechanisms attribute 'catch-slip' bond behavior to the force-induced conformational changes in the lectin-EGF domain hinge of selectins. Below a threshold shear stress, selectins cannot mediate rolling. This 'shear-threshold' phenomenon is a consequence of shear-enhanced tethering and catch bond-enhanced rolling. Quantitative dynamic footprinting microscopy has revealed that leukocytes rolling at venular shear stresses (>0.6 Pa) undergo cellular deformation (large footprint) and form long tethers. The hydrodynamic shear force and torque acting on the rolling cell are thought to be synergistically balanced by the forces acting on tethers and stressed microvilli, however, their relative contribution remains to be determined. Thus, improvement beyond the current understanding requires in silico models that can predict both cellular and microvillus deformation and experiments that allow measurement of forces acting on individual microvilli and tethers.
Figures




References
-
- Addison W. Experimental and practical researches on the structure and function of blood corpuscles; on inflammation, and on the origin and nature of tubercules in the lungs. Trans. Provinc. Med. Surg. Ass. 1843;11:223–306.
-
- Afshar-Kharghan V, Diz-Kucukkaya R, Ludwig EH, et al. Human polymorphism of P-selectin glycoprotein ligand 1 attributable to variable numbers of tandem decameric repeats in the mucinlike region. Blood. 2001;97:3306–3307. - PubMed
-
- Alberts B, Bray D, Lewis J, et al. Molecular biology of the cell. Garland Publishing, Inc.; New York: 1994.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

