Comparison of the Retinomax and Palm-AR Auto-Refractors: a pilot study
- PMID: 21516050
- PMCID: PMC3125429
- DOI: 10.1097/OPX.0b013e3182192658
Comparison of the Retinomax and Palm-AR Auto-Refractors: a pilot study
Abstract
Purpose: To compare the performance of two handheld auto-refractors, the Retinomax and the Palm-Automatic Refractometer (Palm-AR), for detecting significant vision disorders in pre-school children.
Methods: Children attending Philadelphia PreKindergarten Head Start were screened with the Retinomax and Palm-AR and underwent a gold standard eye examination. The results of cycloplegic retinoscopy, cover testing, and visual acuity were used to classify children as having normal vision or one of four conditions: amblyopia, strabismus, significant refractive error, and reduced visual acuity. Pass/fail criteria for each instrument were selected to maximize overall sensitivity (with specificity set at 90% and at 94%) for detecting targeted disorders. Comparisons of sensitivities between the auto-refractors were performed using the exact McNemar test.
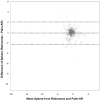
Results: Testability was >99% for both instruments. Test time was similar for the two instruments (median 2 min; p=0.10). At 90% specificity, the sensitivity for detection of one or more targeted conditions was 74% for the Palm-AR and 78% for the Retinomax. At 94% specificity, the sensitivity for detection of one or more targeted conditions was 66% for both the Palm-AR and the Retinomax. At 90% specificity, the sensitivity for detecting significant refractive error was 84% for both auto-refractors, and at 94% specificity, the sensitivity was 76% for the Palm AR and 75% for the Retinomax. There were high correlations between the instruments for sphere (r=0.85) and cylinder (r=0.88) power. The mean difference between instruments was -0.13 diopters (D) (95% limit of agreement: -2.28 to 2.02) for sphere, and -0.15 D (95% limit of agreement: -0.89 to 0.59) for cylinder.
Conclusions: In this pilot study, the Retinomax and Palm-AR appear comparable with respect to testability, sensitivity, and specificity. There was strong agreement in readings of sphere and cylinder indicating that they may perform similarly in a screening setting.
Copyright © 2011 American Academy of Optometry
Figures

References
-
- U.S. Public Health Service. Vision screening in children. Am Fam Physician. 1994;50:587–90. - PubMed
-
- Moore B. Eye Care for Infants and Young Children. Boston: Butterworth-Heinemann; 1997.
-
- Ciner EB, Schmidt PP, Orel-Bixler D, Dobson V, Maguire M, Cyert L, Moore B, Schultz J. Vision screening of preschool children: evaluating the past, looking toward the future. Optom Vis Sci. 1998;75:571–84. - PubMed
-
- American Optometric Association Consensus Panel on Pediatric Eye and Vision Examination. Pediatric Eye and Vision Examinations. 2. St. Louis: American Optometric Association; 2002. - PubMed
-
- American Academy of Ophthalmology Preferred Practice Patterns Pediatric Ophthalmology Panel. Preferred Practice Pattern. San Francisco: American Academy of Ophthalmology; 2002. Pediatric Eye Evaluations.
Publication types
MeSH terms
Substances
Grants and funding
- U10EY12534/EY/NEI NIH HHS/United States
- U10 EY012545/EY/NEI NIH HHS/United States
- R21 EY018908/EY/NEI NIH HHS/United States
- U10EY12545/EY/NEI NIH HHS/United States
- U10 EY012647/EY/NEI NIH HHS/United States
- U10 EY012648/EY/NEI NIH HHS/United States
- U10 EY012550/EY/NEI NIH HHS/United States
- U10EY12550/EY/NEI NIH HHS/United States
- U10 EY012644/EY/NEI NIH HHS/United States
- U10 EY012534/EY/NEI NIH HHS/United States
- R21EY018908/EY/NEI NIH HHS/United States
- U10EY12545-04S1/EY/NEI NIH HHS/United States
- U10EY12547/EY/NEI NIH HHS/United States
- U10EY12644/EY/NEI NIH HHS/United States
- U10EY12648/EY/NEI NIH HHS/United States
- U10EY12647/EY/NEI NIH HHS/United States
- U10 EY012547/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

