Conformational changes in IgE contribute to its uniquely slow dissociation rate from receptor FcɛRI
- PMID: 21516097
- PMCID: PMC3357048
- DOI: 10.1038/nsmb.2044
Conformational changes in IgE contribute to its uniquely slow dissociation rate from receptor FcɛRI
Abstract
Among antibody classes, IgE has a uniquely slow dissociation rate from, and high affinity for, its cell surface receptor FcɛRI. We show the structural basis for these key determinants of the ability of IgE to mediate allergic hypersensitivity through the 3.4-Å-resolution crystal structure of human IgE-Fc (consisting of the Cɛ2, Cɛ3 and Cɛ4 domains) bound to the extracellular domains of the FcɛRI α chain. Comparison with the structure of free IgE-Fc (reported here at a resolution of 1.9 Å) shows that the antibody, which has a compact, bent structure before receptor engagement, becomes even more acutely bent in the complex. Thermodynamic analysis indicates that the interaction is entropically driven, which explains how the noncontacting Cɛ2 domains, in place of the flexible hinge region of IgG antibodies, contribute together with the conformational changes to the unique binding properties of IgE.
Figures
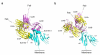



References
-
- Asher MI, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–743. - PubMed
-
- Holgate S, et al. The use of omalizumab in the treatment of severe allergic asthma: A clinical experience update. Respir. Med. 2009;103:1098–1113. - PubMed
-
- Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nature Rev. Immunol. 2008;8:205–217. - PubMed
-
- Maenaka K, van der Merwe PA, Stuart DI, Jones EY, Sondermann P. The human low affinity Fcγ receptors IIa, IIb and III bind IgG with fast kinetics and distinct thermodynamic properties. J. Biol. Chem. 2001;276:44898–44904. - PubMed
-
- Gould HJ, et al. The biology of IgE and the basis of allergic disease. Ann. Rev. Immunol. 2003;21:579–628. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
- G0701506/MRC_/Medical Research Council/United Kingdom
- G0200486/MRC_/Medical Research Council/United Kingdom
- G0501494(76353)/MRC_/Medical Research Council/United Kingdom
- G1000758/MRC_/Medical Research Council/United Kingdom
- 090532/WT_/Wellcome Trust/United Kingdom
- G0501494/MRC_/Medical Research Council/United Kingdom
- G0601043/MRC_/Medical Research Council/United Kingdom
- G0200486(63136)/MRC_/Medical Research Council/United Kingdom
- MC_G0900865/MRC_/Medical Research Council/United Kingdom
- G0001352/MRC_/Medical Research Council/United Kingdom
- 081320/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources

