The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF
- PMID: 21516111
- PMCID: PMC3116521
- DOI: 10.1038/ni.2031
The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF
Abstract
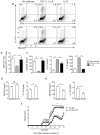
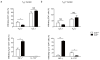
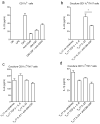
Interleukin 17 (IL-17)-producing helper T cells (T(H)17 cells) require exposure to IL-23 to become encephalitogenic, but the mechanism by which IL-23 promotes their pathogenicity is not known. Here we found that IL-23 induced production of the cytokine granulocyte-macrophage colony-stimulating factor (GM-CSF) in T(H)17 cells and that GM-CSF had an essential role in their encephalitogenicity. Our findings identify a chief mechanism that underlies the important role of IL-23 in autoimmune diseases. IL-23 induced a positive feedback loop whereby GM-CSF secreted by T(H)17 cells stimulated the production of IL-23 by antigen-presenting cells. Such cross-regulation of IL-23 and GM-CSF explains the similar pattern of resistance to autoimmunity when either of the two cytokines is absent and identifies T(H)17 cells as a crucial source of GM-CSF in autoimmune inflammation.
Figures




Comment in
-
GM-CSF: the secret weapon in the T(H)17 arsenal.Nat Immunol. 2011 Jun;12(6):521-2. doi: 10.1038/ni.2044. Nat Immunol. 2011. PMID: 21587311
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

