The role of the myosin ATPase activity in adaptive thermogenesis by skeletal muscle
- PMID: 21516138
- PMCID: PMC3064898
- DOI: 10.1007/s12551-011-0044-9
The role of the myosin ATPase activity in adaptive thermogenesis by skeletal muscle
Abstract
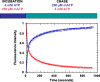
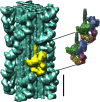
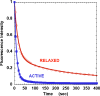
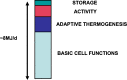
Resting skeletal muscle is a major contributor to adaptive thermogenesis, i.e., the thermogenesis that changes in response to exposure to cold or to overfeeding. The identification of the "furnace" that is responsible for increased heat generation in resting muscle has been the subject of a number of investigations. A new state of myosin, the super relaxed state (SRX), with a very slow ATP turnover rate has recently been observed in skeletal muscle (Stewart et al. in Proc Natl Acad Sci USA 107:430-435, 2010). Inhibition of the myosin ATPase activity in the SRX was suggested to be caused by binding of the myosin head to the core of the thick filament in a structural motif identified earlier by electron microscopy. To be compatible with the basal metabolic rate observed in vivo for resting muscle, most myosin heads would have to be in the SRX. Modulation of the population of this state, relative to the normal relaxed state, was proposed to be a major contributor to adaptive thermogenesis in resting muscle. Transfer of only 20% of myosin heads from the SRX into the normal relaxed state would cause muscle thermogenesis to double. Phosphorylation of the myosin regulatory light chain was shown to transfer myosin heads from the SRX into the relaxed state, which would increase thermogenesis. In particular, thermogenesis by myosin has been proposed to play a role in the dissipation of calories during overfeeding. Up-regulation of muscle thermogenesis by pharmaceuticals that target the SRX would provide new approaches to the treatment of obesity or high blood sugar levels.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

