Intracoronary administration of cardiac stem cells in mice: a new, improved technique for cell therapy in murine models
- PMID: 21516491
- PMCID: PMC3640450
- DOI: 10.1007/s00395-011-0180-1
Intracoronary administration of cardiac stem cells in mice: a new, improved technique for cell therapy in murine models
Abstract
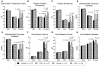
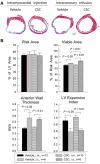
A model of intracoronary stem cell delivery that enables transgenesis/gene targeting would be a powerful tool but is still lacking. To address this gap, we compared intracoronary and intramyocardial delivery of lin(-)/c-kit(+)/GFP(+) cardiac stem cells (CSCs) in a murine model of reperfused myocardial infarction (MI). Lin(-)/c-kit(+)/GFP(+) CSCs were successfully expanded from GFP transgenic hearts and cultured with no detectable phenotypic change for up to ten passages. Intracoronary delivery of CSCs 2 days post-MI resulted in significant alleviation of adverse LV remodeling and dysfunction, which was at least equivalent, if not superior, to that achieved with intramyocardial delivery. Compared with intramyocardial injection, intracoronary infusion was associated with a more homogeneous distribution of CSCs in the infarcted region and a greater increase in viable tissue in this region, suggesting greater formation of new cardiomyocytes. Intracoronary CSC delivery resulted in improved function in the infarcted region, as well as in improved global LV systolic and diastolic function, and in decreased LV dilation and LV expansion index; the magnitude of these effects was similar to that observed after intramyocardial injection. We conclude that, in the murine model of reperfused MI, intracoronary CSC infusion is at least as effective as intramyocardial injection in limiting LV remodeling and improving both regional and global LV function. The intracoronary route appears to be superior in terms of uniformity of cell distribution, myocyte regeneration, and amount of viable tissue in the risk region. To our knowledge, this is the first study to report that intracoronary infusion of stem cells in mice is feasible and effective.
Figures




References
-
- Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D’Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci USA. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. - DOI - PMC - PubMed
-
- Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

